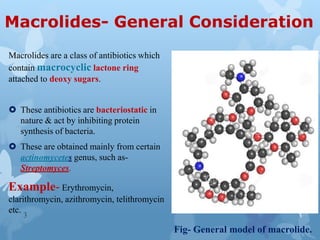
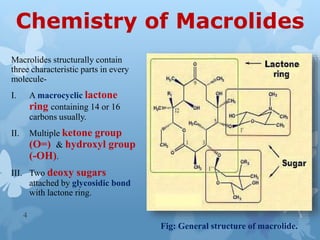
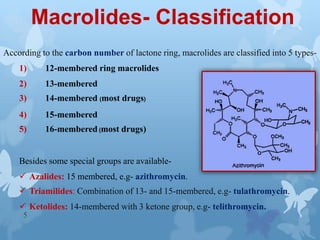
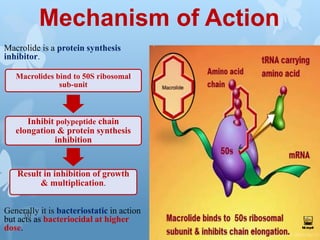
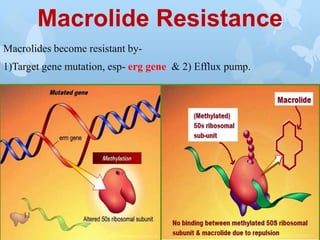
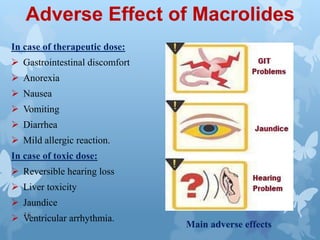
The document discusses macrolide antibiotics. It describes macrolides as a class of antibiotics that contain a macrocyclic lactone ring attached to deoxy sugars. They are bacteriostatic and inhibit bacterial protein synthesis. Macrolides are classified based on the number of carbons in their lactone ring and examples include erythromycin, clarithromycin, and azithromycin. Macrolides are absorbed orally and distributed widely throughout the body, metabolized in the liver, and excreted primarily in bile. Their mechanisms of action, resistance, spectrum of activity, indications, contraindications and adverse effects are also summarized.