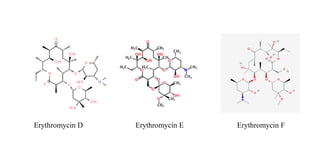
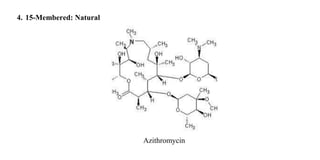
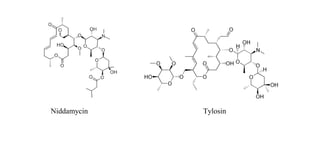
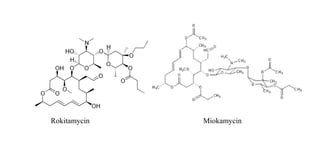
Macrolide antibiotics are a class of antibiotics that contain a macrocyclic lactone ring attached to deoxy sugars. They are usually obtained from actinomycetes bacteria and are bacteriostatic, inhibiting bacterial protein synthesis by binding to the 50S ribosomal subunit. First-line uses include treating atypical pneumonia, H. pylori, and allergies to penicillin. Later discoveries expanded the ring size and added groups to increase stability, spectrum, and pharmacokinetics. Macrolides degrade under acidic conditions through an intramolecular reaction forming an inactive ketal.