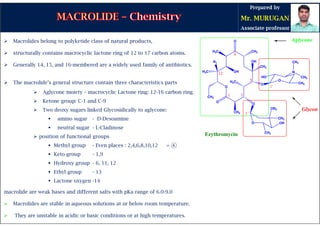
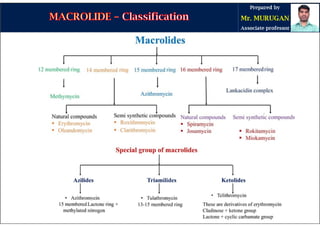
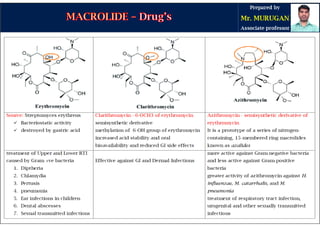
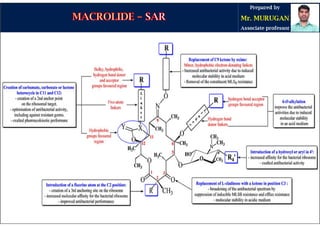
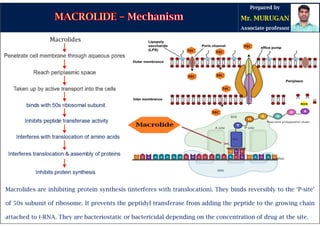
The document provides an overview of macrolide antibiotics, detailing their historical development, chemical structure, and classification. Key macrolides like erythromycin, clarithromycin, and azithromycin are discussed in terms of their mechanism of action, therapeutic uses, and side effects. It also covers the evolution of these antibiotics and their clinical applications, highlighting their effectiveness against various bacterial infections.