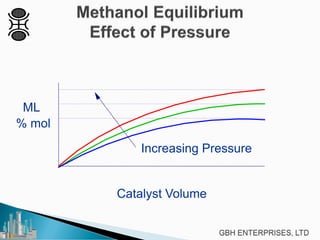
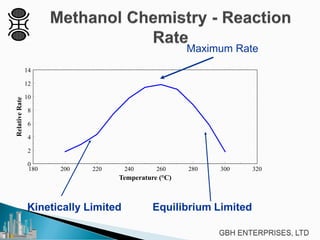
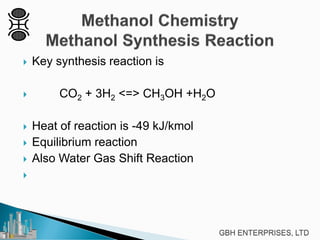
This document discusses methanol synthesis from carbon dioxide and hydrogen. It provides details on the key synthesis reaction, kinetics, equilibrium, catalyst activity, byproducts and side reactions. The maximum rate of the methanol reaction is defined and diagrams show how operating conditions can be optimized to follow the maximum rate line for minimum catalyst volume. Potential byproducts like ethanol, ketones, and hydrocarbons are also summarized.



![ The reaction rate is defined by the following
equation
−=
1
3
22
3
22
3
1
KHPCOP
OHCHP
HPCOPkp
dt
OHCHd mn
.][].[
][
.][.][.
][
Where P[] is the partial pressure
Kinetic Term Equilibrium Term](https://image.slidesharecdn.com/methanolsynthesischemistry-130730213610-phpapp01/85/Methanol-Synthesis-Chemistry-5-320.jpg)

![ The reaction rate is defined by the following
equation
−=
1
2
2
3
2
3
.][].[
][
1.][.][.
][
KHPCOP
OHCHP
HPCOPkp
dt
OHCHd mn
Where P[] is the partial pressure
Kinetic Term Equilibrium Term](https://image.slidesharecdn.com/methanolsynthesischemistry-130730213610-phpapp01/85/Methanol-Synthesis-Chemistry-7-320.jpg)