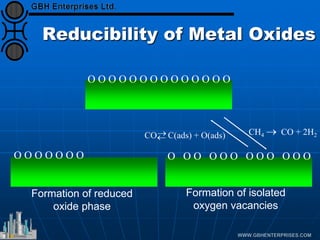
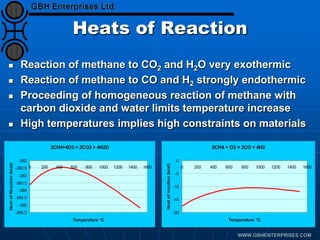
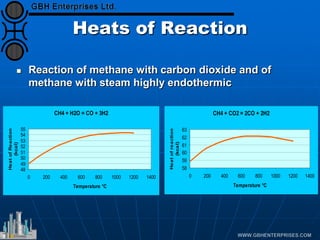
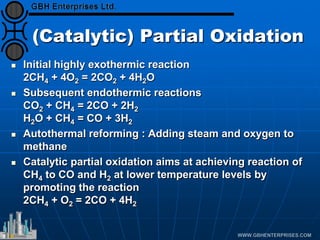
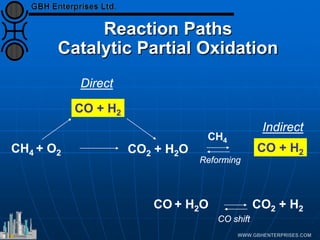
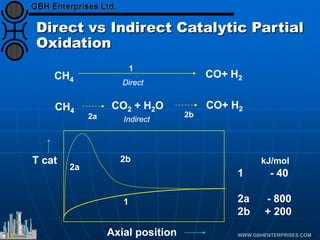
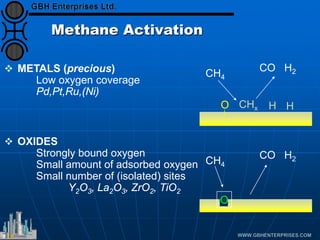
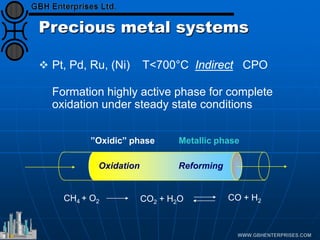
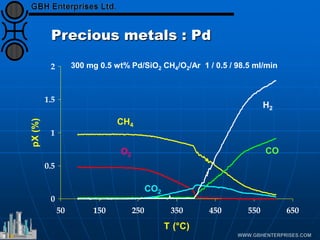
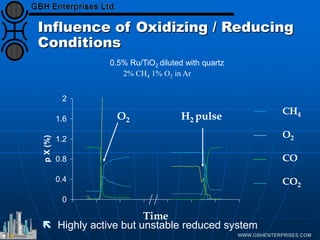
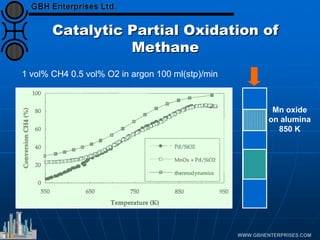
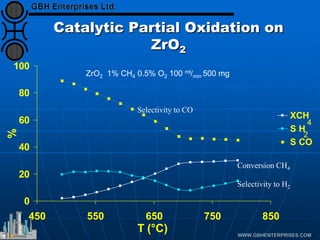
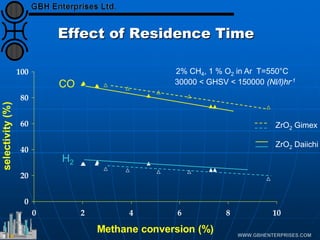
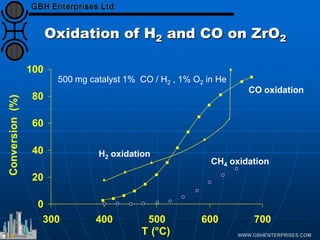
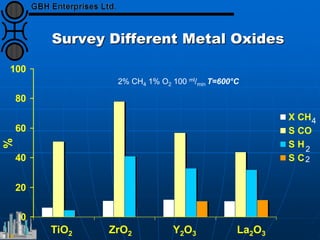
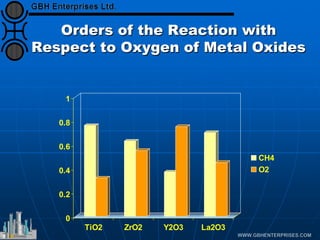
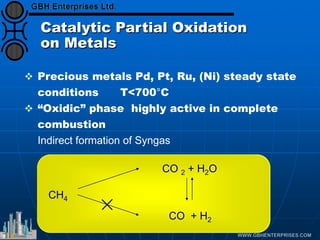
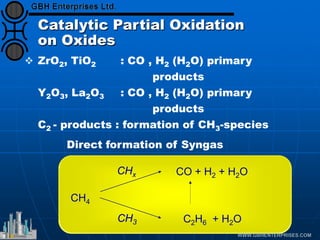
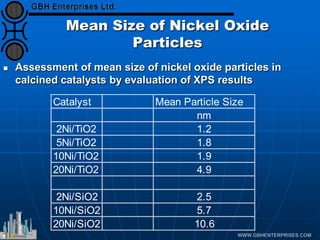
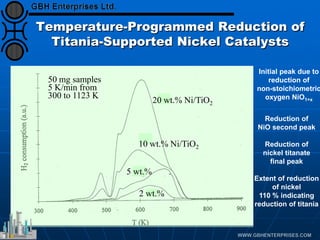
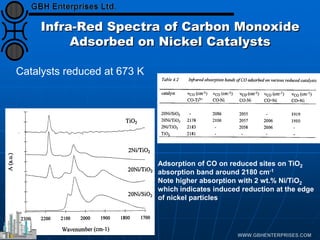
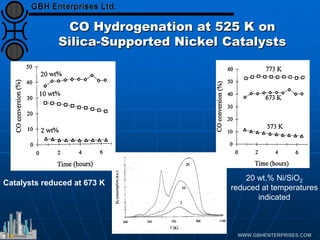
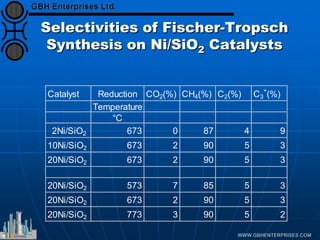
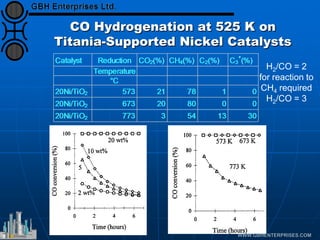
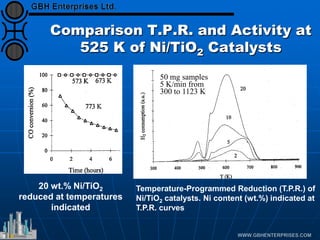
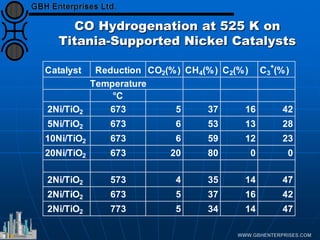
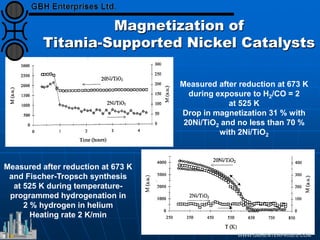
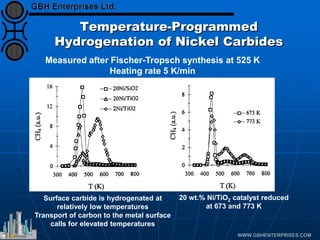
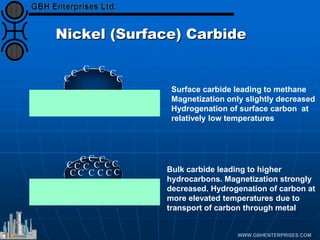
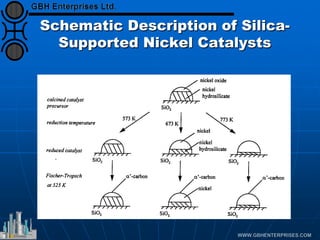
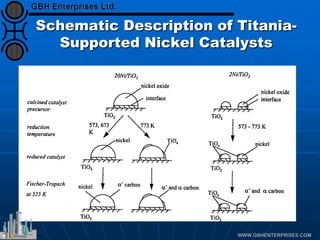
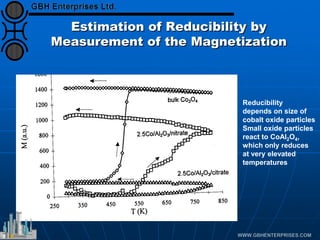
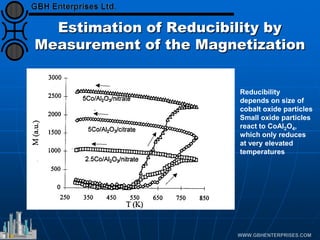
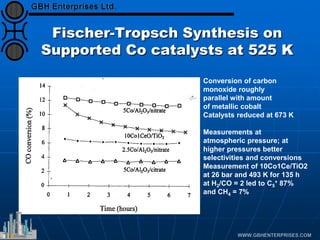
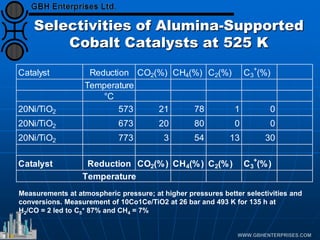
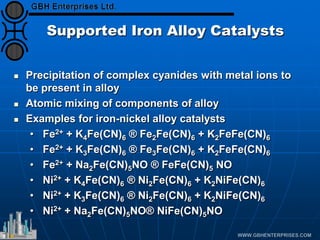
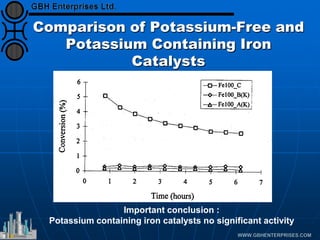
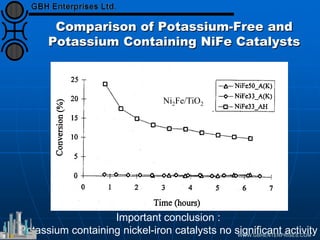
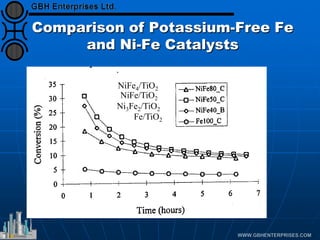
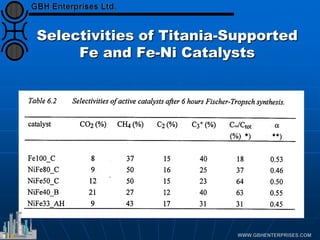
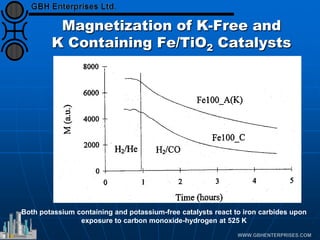
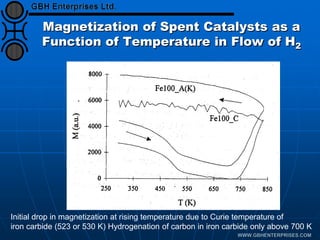
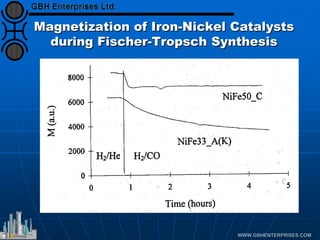
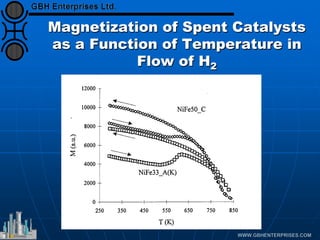
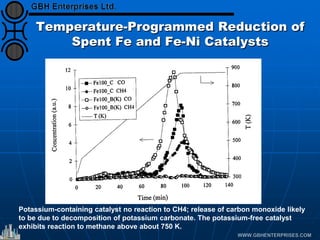
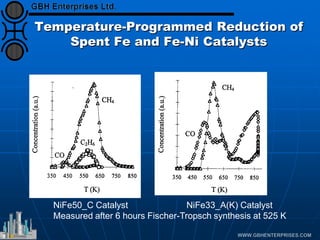
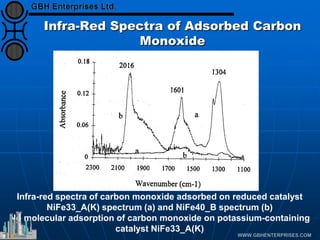
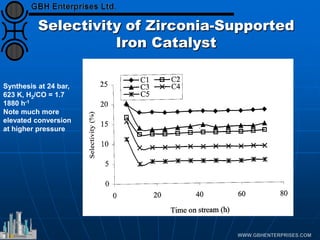
The document discusses catalyst preparation and behavior in catalytic reactions such as Fischer-Tropsch synthesis and catalytic partial oxidation. It describes the Fischer-Tropsch process, different catalyst types including precious metals and metal oxides, and preparation methods like deposition-precipitation. Temperature-programmed reduction is used to analyze the reducibility of nickel oxide catalysts supported on silica and titania. The document provides details on catalyst characterization and evaluation.