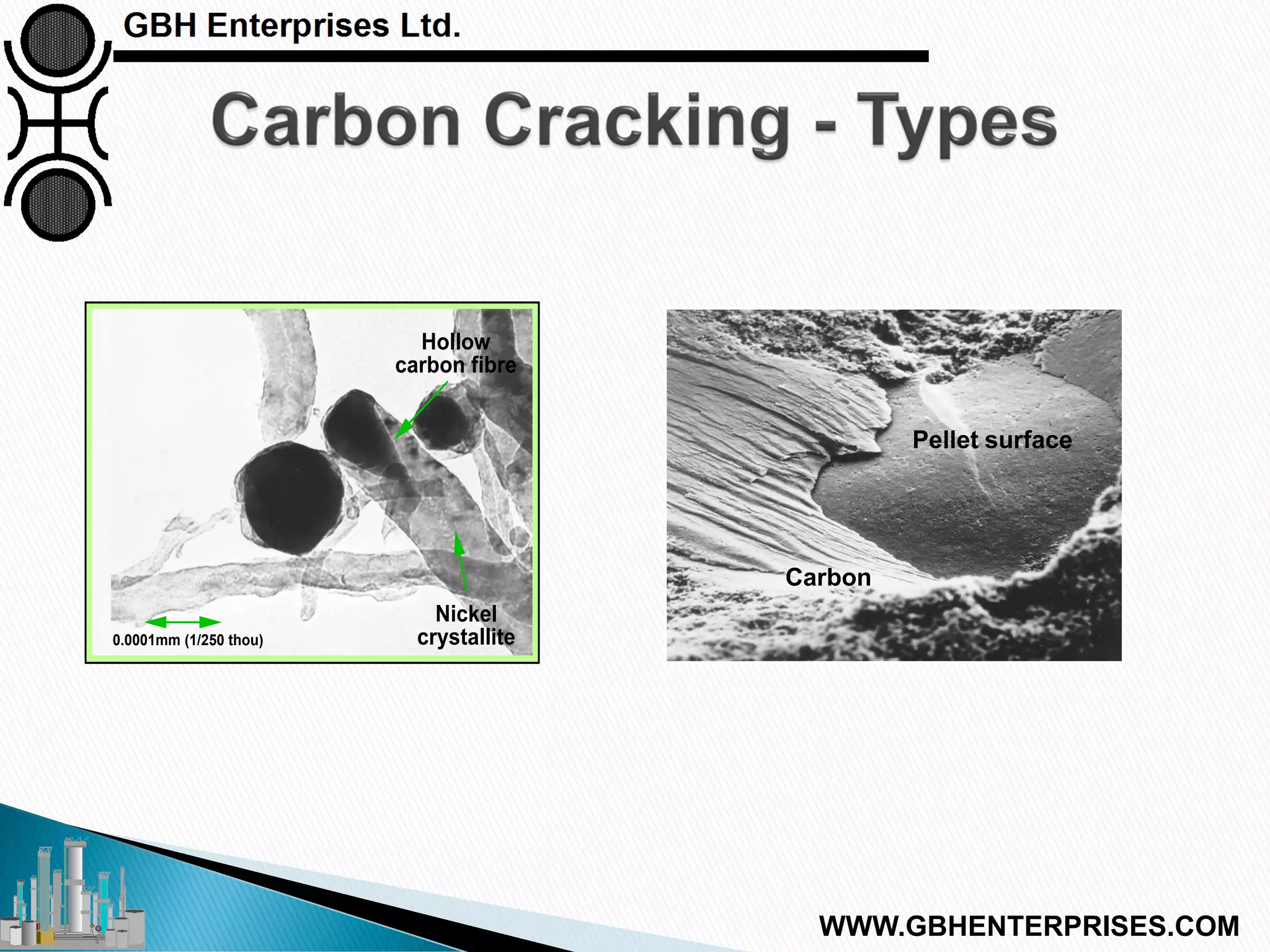
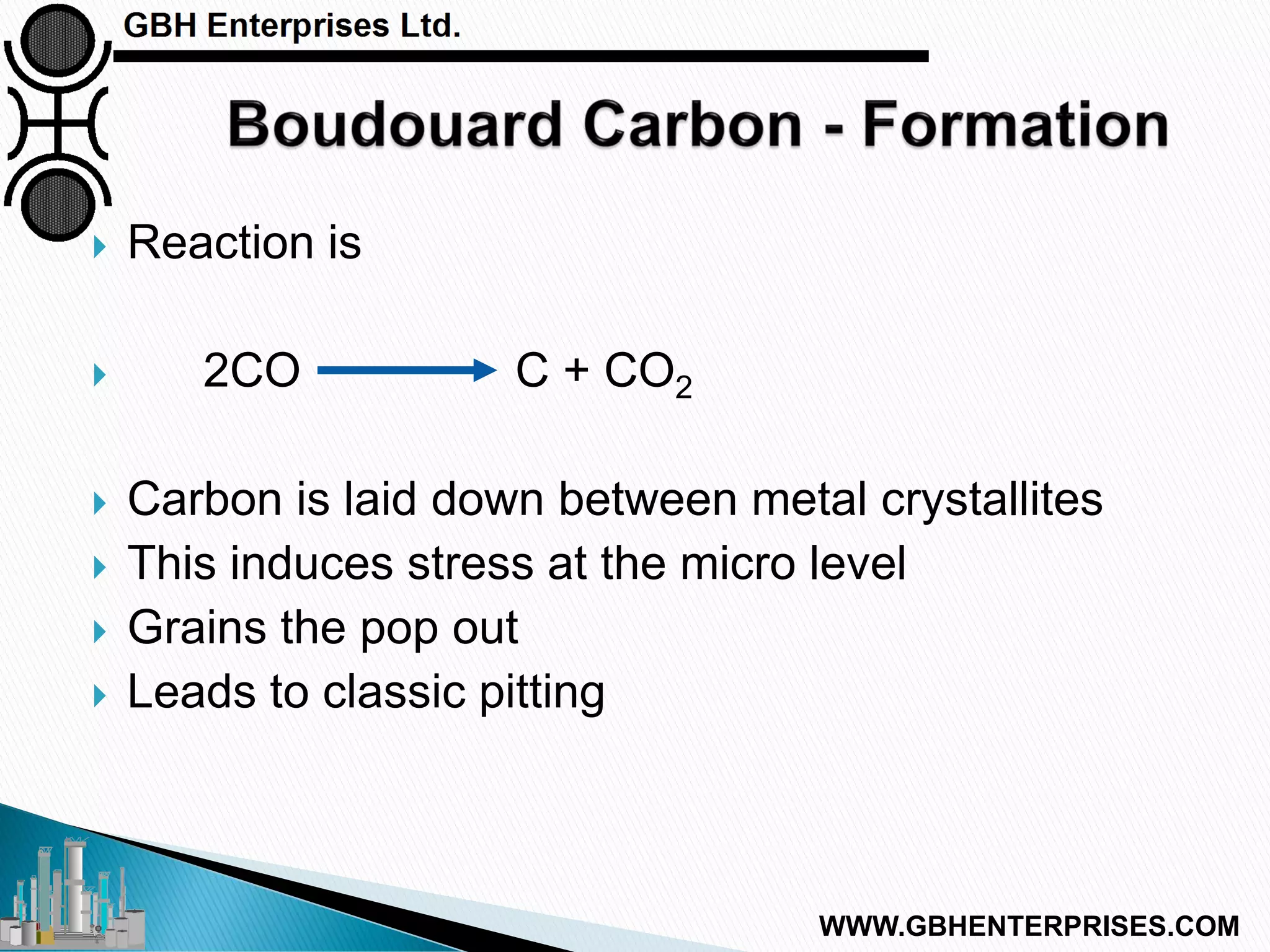
The presentation by Gerard B. Hawkins aims to explain carbon formation in catalytic processes, emphasizing the reasons, types, and prevention methods for carbon-related issues. It identifies three main types of carbon: cracking, Boudouard, and CO reduction, with a focus on cracking due to its prevalence. The document discusses the conditions that lead to carbon formation and suggests strategies for mitigating its impact on catalyst performance.