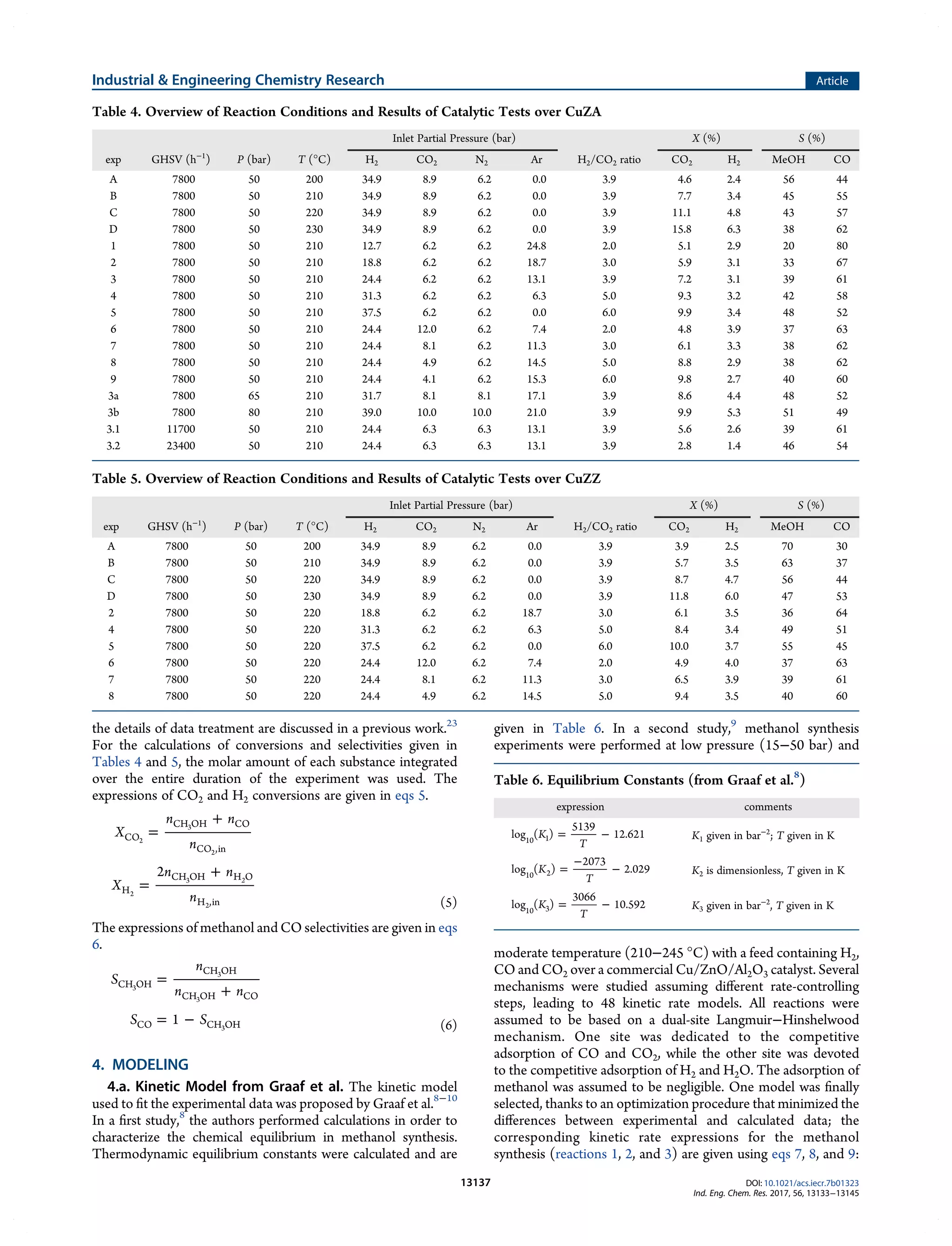
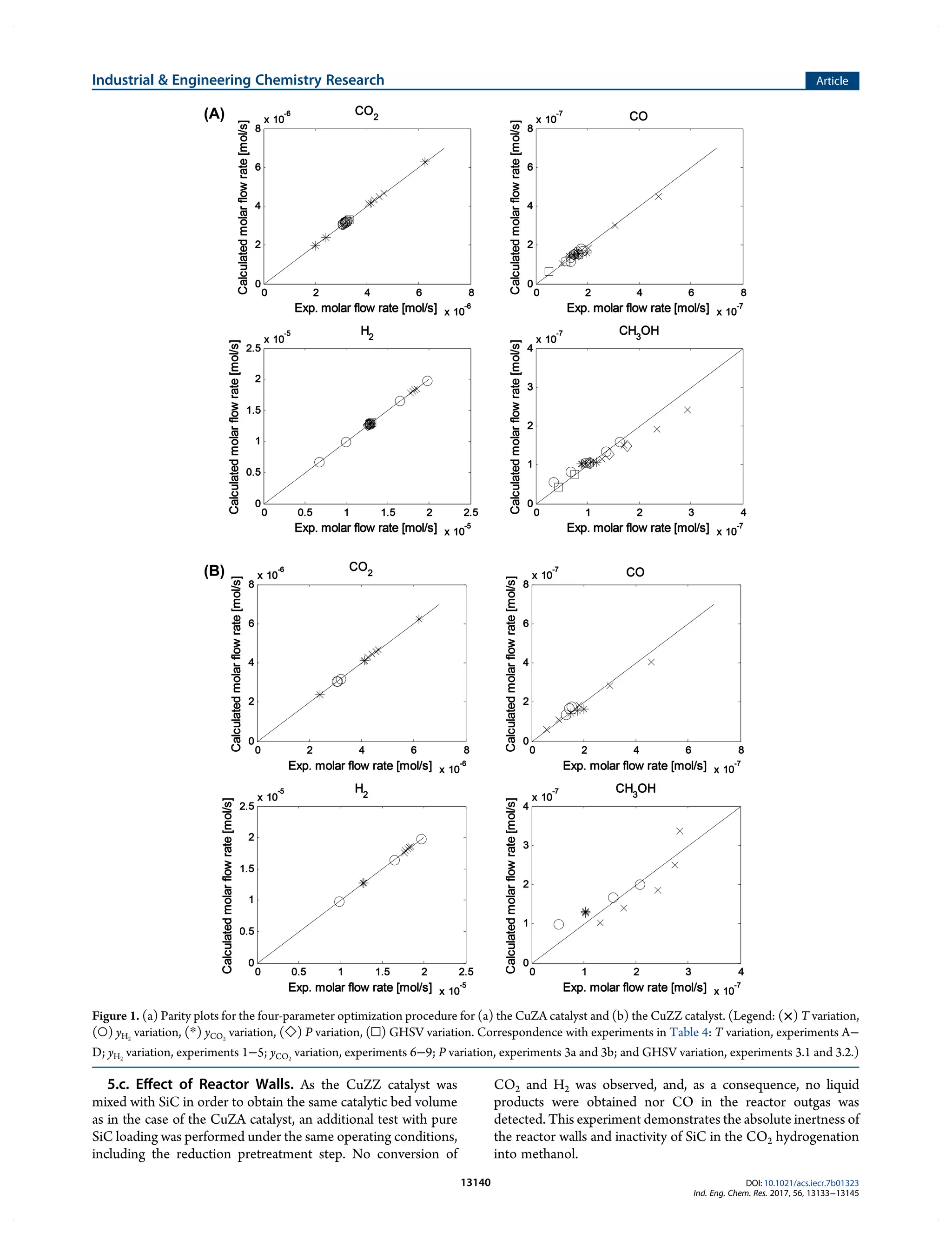
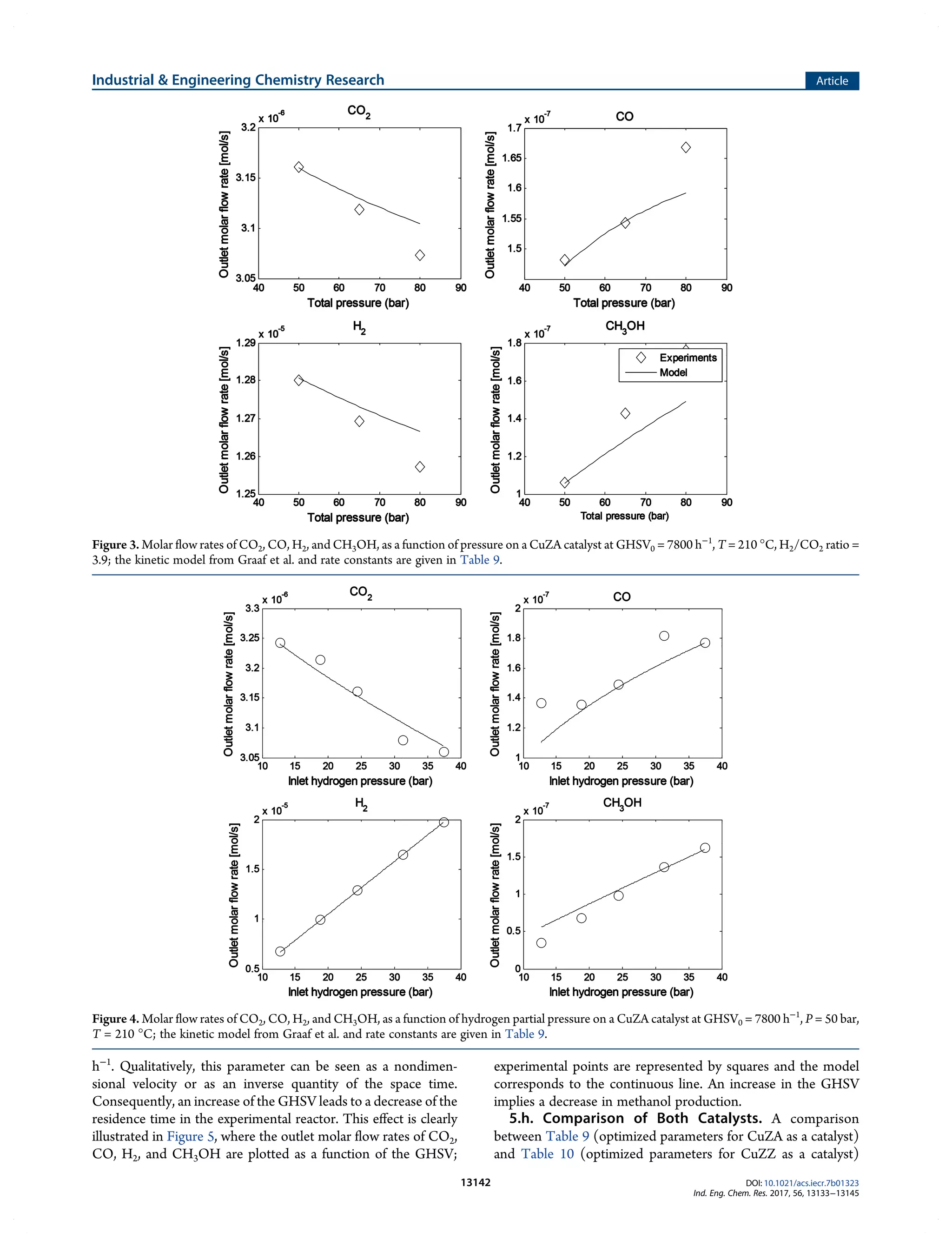
This document summarizes a study on the kinetics of methanol synthesis from carbon dioxide hydrogenation over copper-zinc oxide catalysts. Experiments were conducted in a fixed bed reactor between 200-230°C, 50-80 bar, and gas hourly space velocities of 7,800-23,400 h-1 using feeds with H2:CO2 ratios of 2-6 without CO. Kinetic parameters from a previous study were optimized to model the experimental data using a Langmuir–Hinshelwood–Hougen–Watson mechanism. The influences of catalyst support (alumina vs zirconia) and operating conditions on kinetics were examined. The goal was to determine optimized parameters to reliably scale-up the