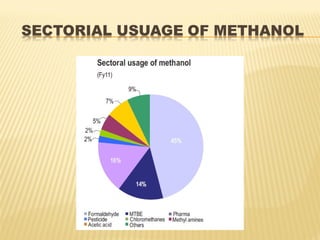
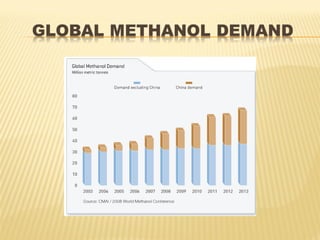
Methanol is the simplest alcohol and can be used as an alternative fuel or chemical feedstock. It is produced via a four step process: feed purification using desulphurization; steam reforming of natural gas over nickel catalysts at high pressures and temperatures; methanol synthesis over copper catalysts in a reactor; and methanol purification through distillation. Methanol production facilities are located globally and the demand for methanol is increasing in countries like India at 7-8% annually.