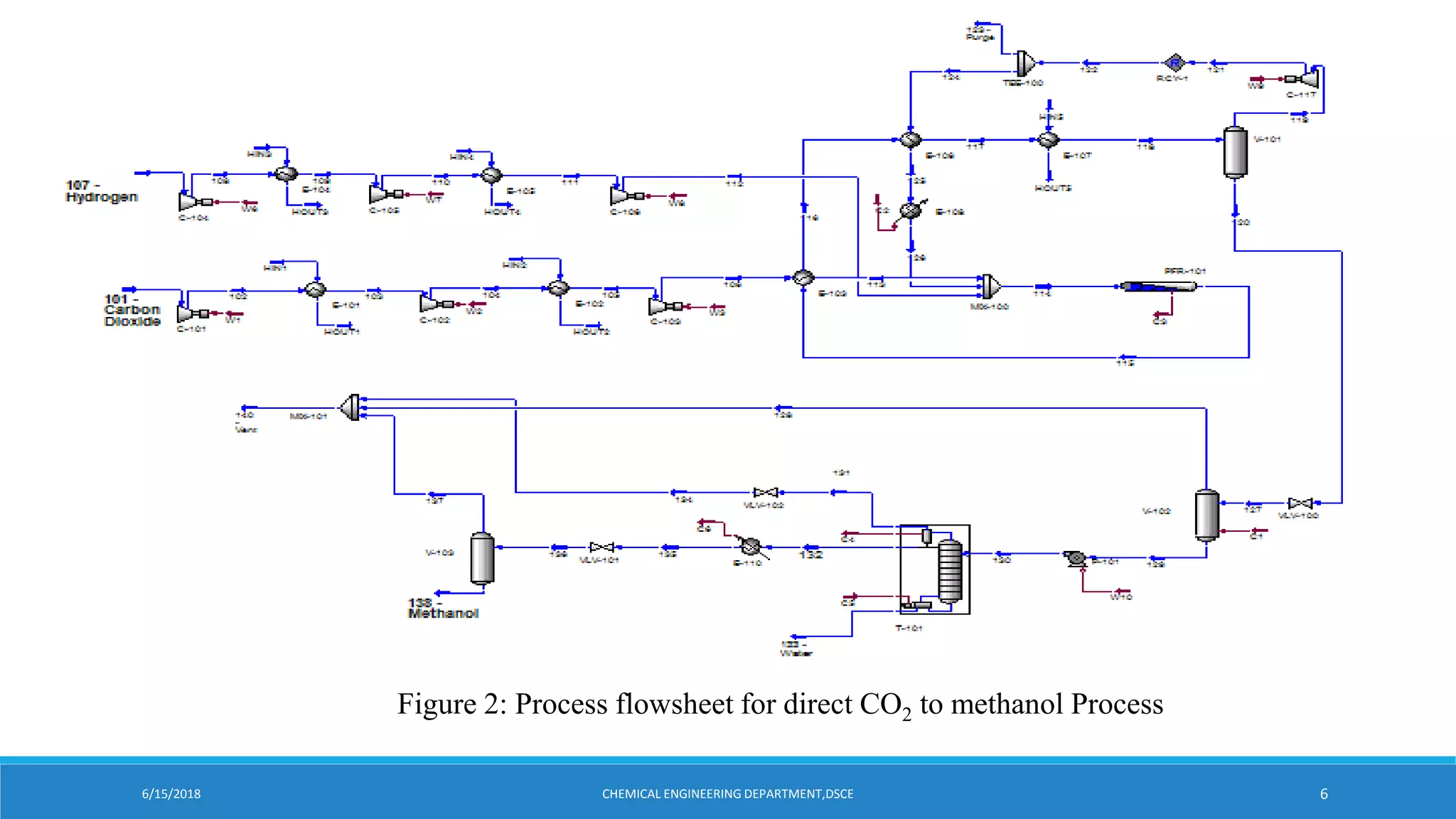
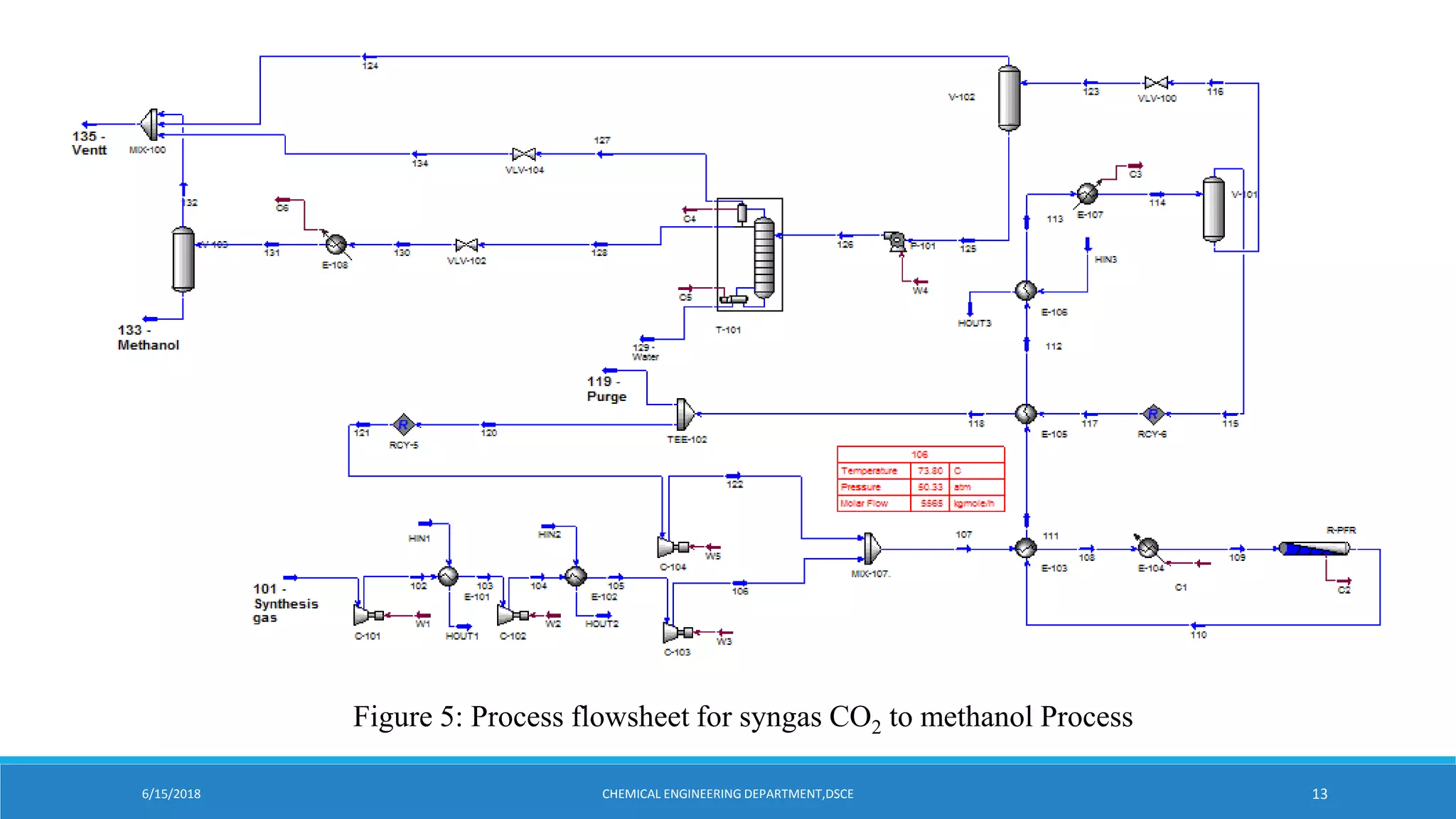
Kishan Kasundra presented on methanol synthesis from industrial CO2 sources. Two case studies were analyzed: direct CO2 to methanol (d-CTM) and synthesis gas to methanol (sg-CTM). The d-CTM process consumed more utilities and CO2 per ton of methanol but emitted slightly more CO2. The sg-CTM process optimized methanol production at high hydrogen-carbon ratios and was less resource intensive. Both achieved high methanol yields but differed in raw material use and carbon emissions. The presentation concluded the sg-CTM route may be preferable due to lower resource use and carbon emissions per ton of methanol produced.