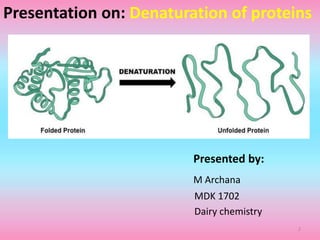
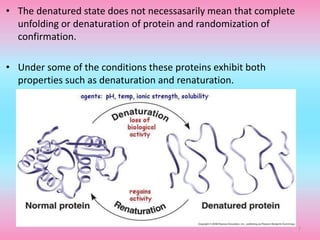
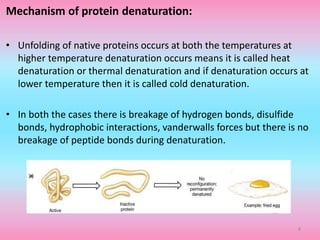
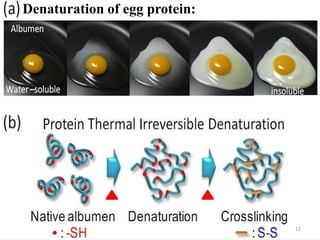
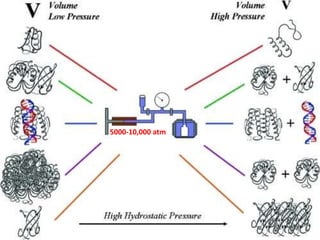
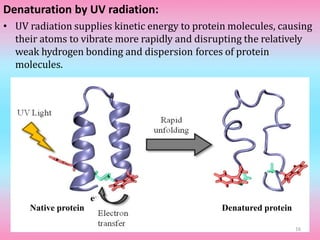
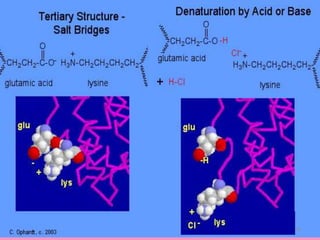
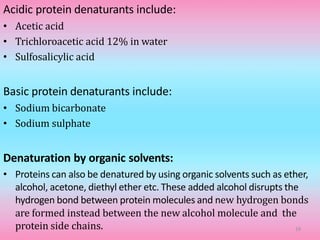
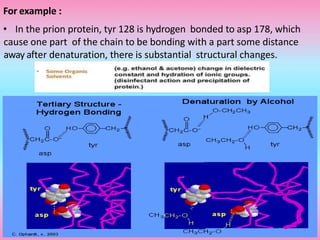
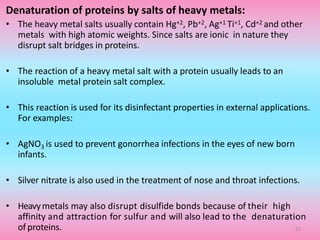
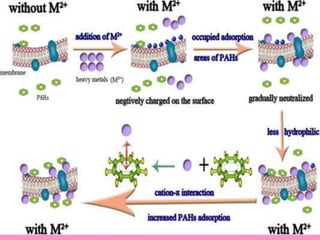
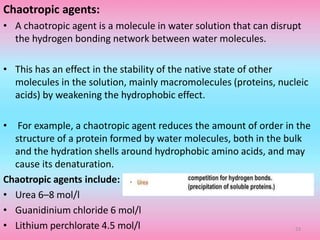
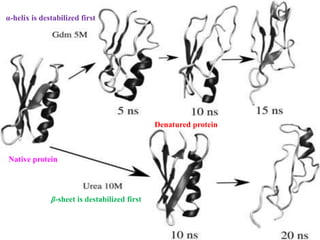
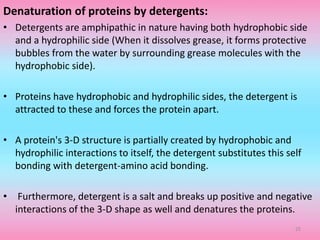
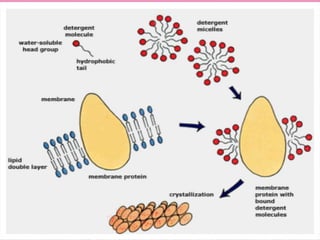
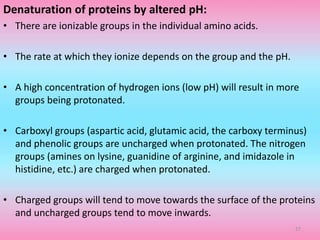
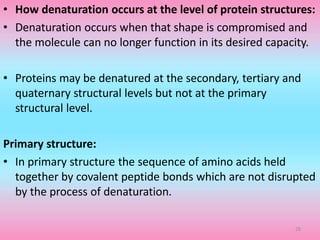
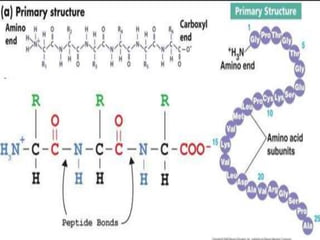
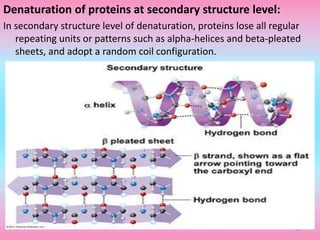
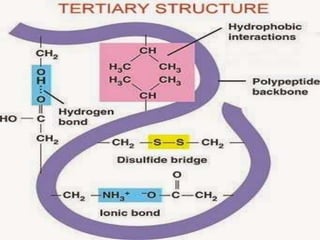
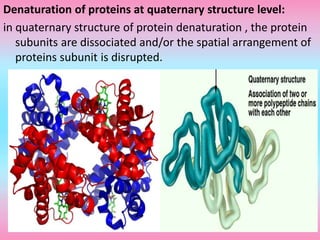
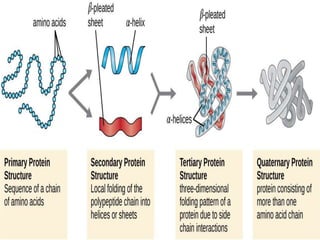
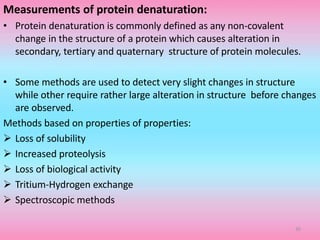
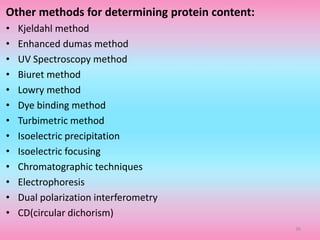
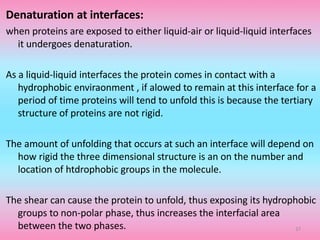
The document discusses protein denaturation, a process wherein proteins lose their native structure and biological activity due to various physical and chemical agents, such as heat, pH changes, and agitation. It outlines the mechanisms of denaturation, the agents involved, and the implications of denaturation on protein function. The summary also highlights the advantages and disadvantages of protein denaturation, particularly in contexts such as food chemistry and proteomics.