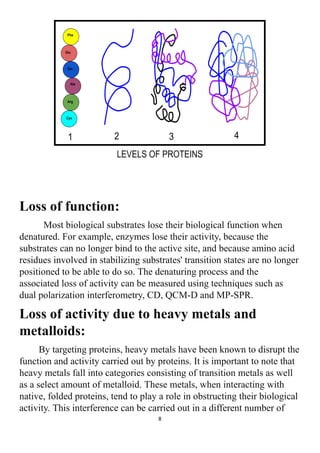
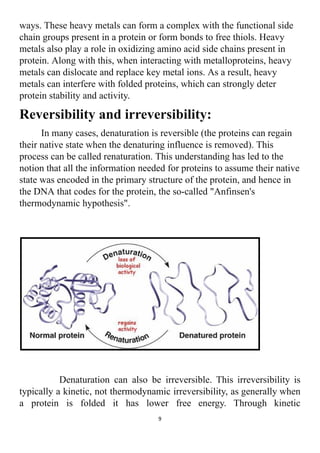
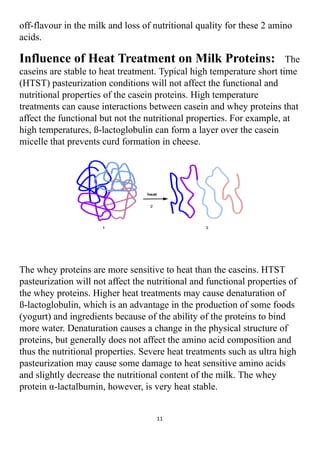
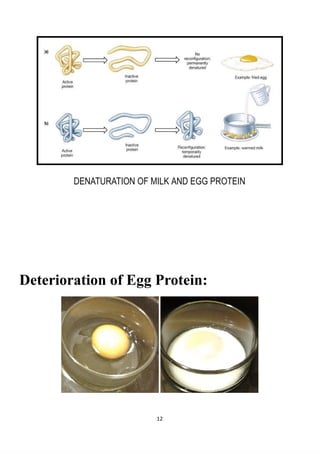
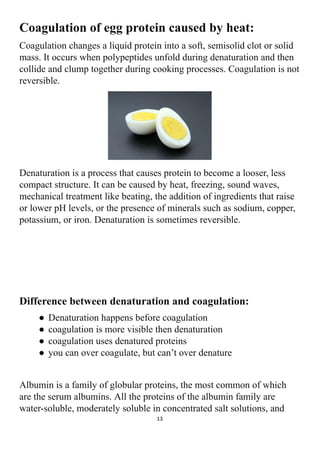
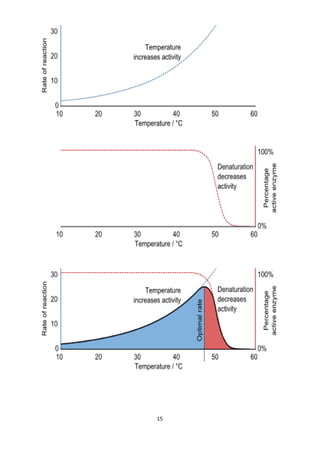
The project explores the denaturation of milk and egg proteins, detailing the effects of heat, pH, and external factors on their structure and function. Key findings show that egg protein denatures at 36°C while milk protein denatures at 83°C, indicating that different proteins have unique thermal stability. Acknowledgments highlight the contributions of teachers, family, and peers in completing the research.