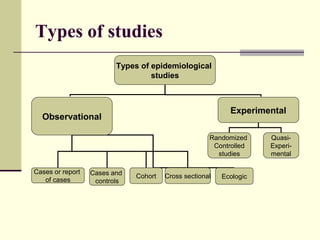
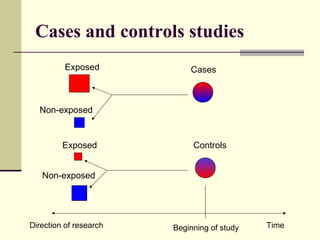
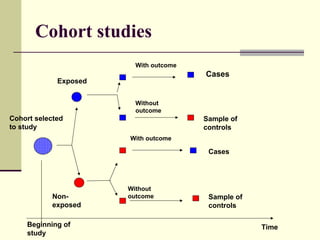
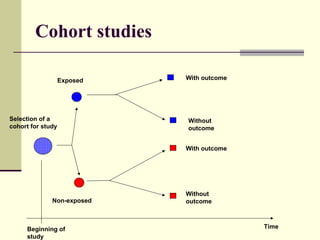
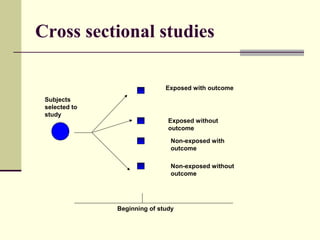
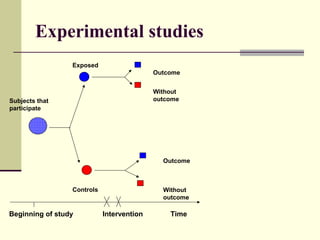
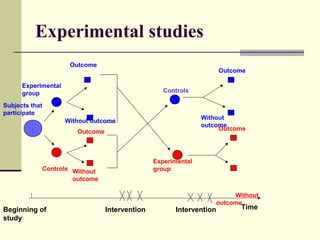
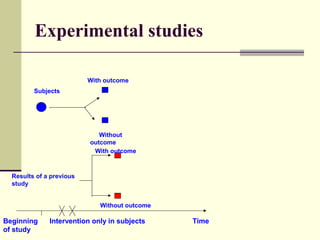
This document describes different types of epidemiological studies, including observational studies like case reports, case-control studies, cohort studies, and cross-sectional studies. It also describes experimental studies like randomized controlled trials and quasi-experimental studies. For each type of study, it provides brief descriptions and notes advantages and disadvantages.