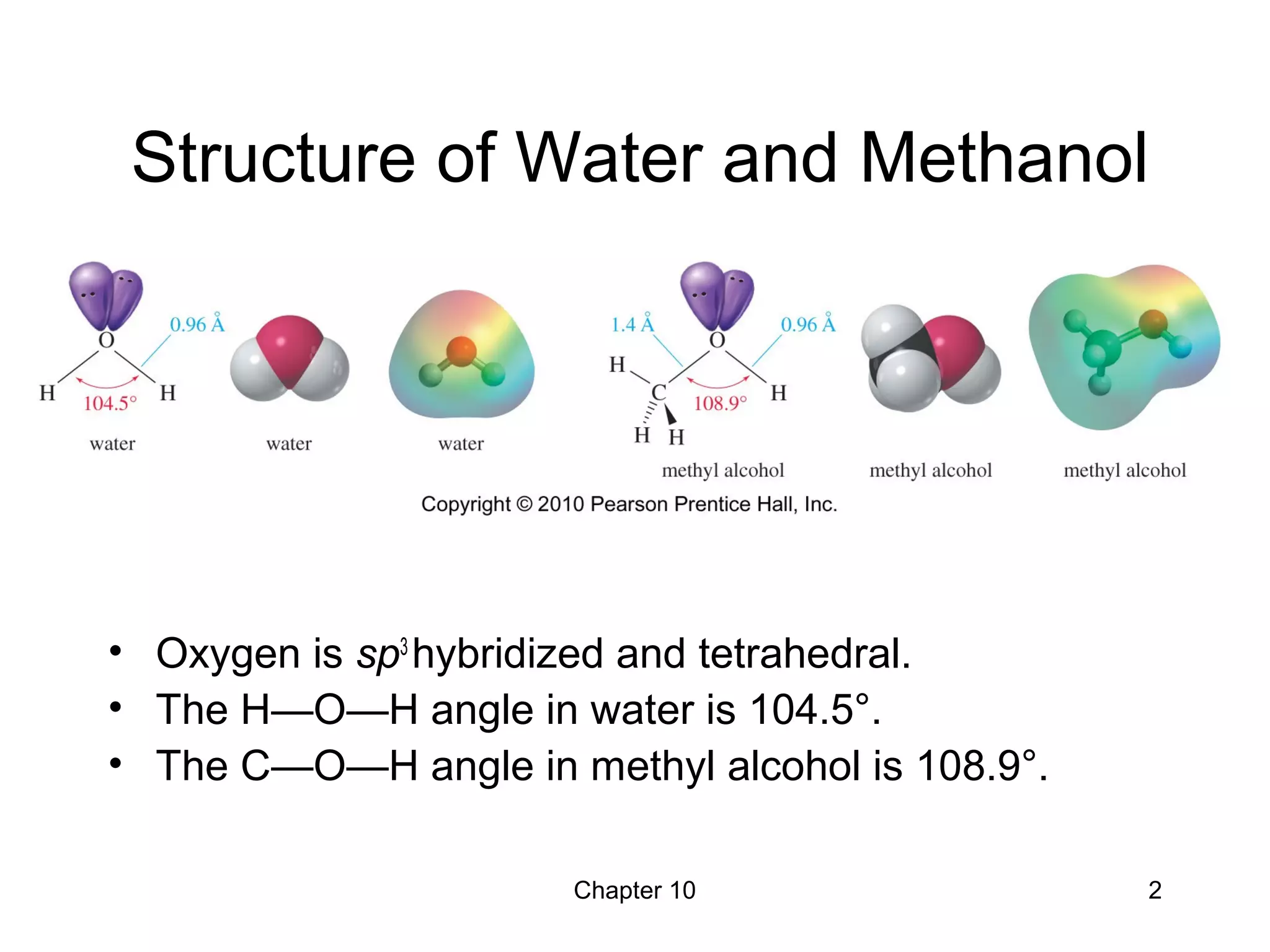
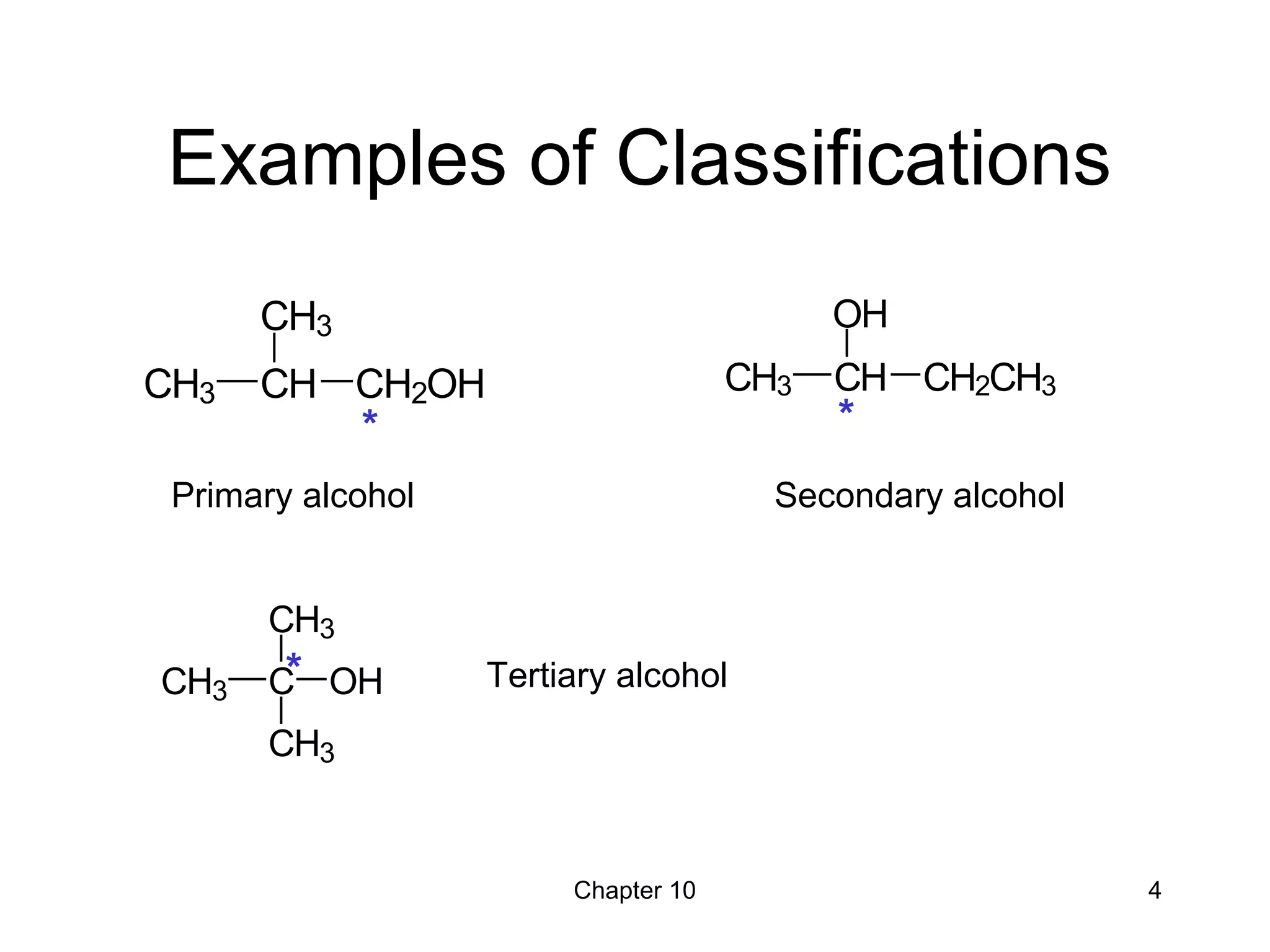
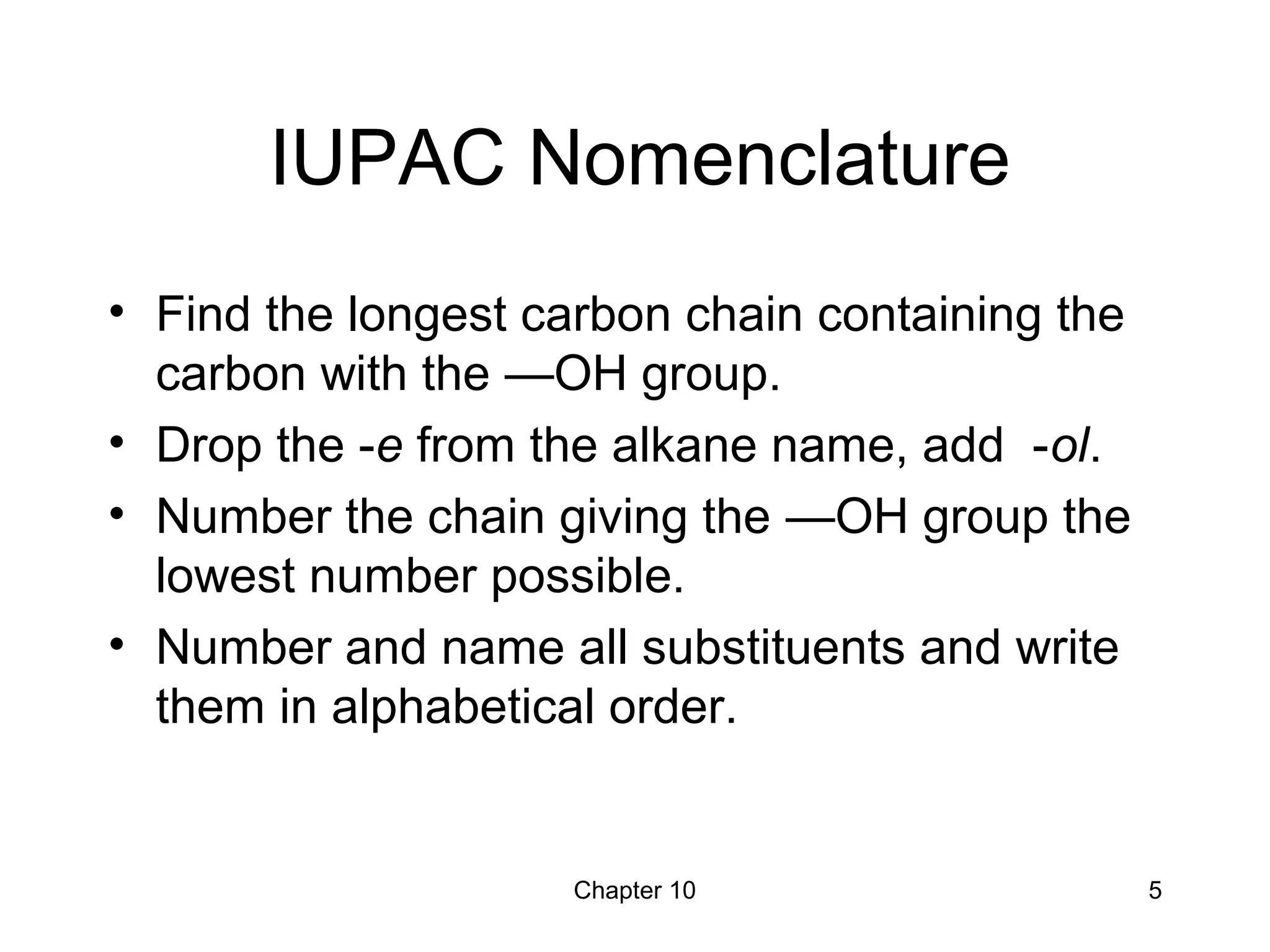
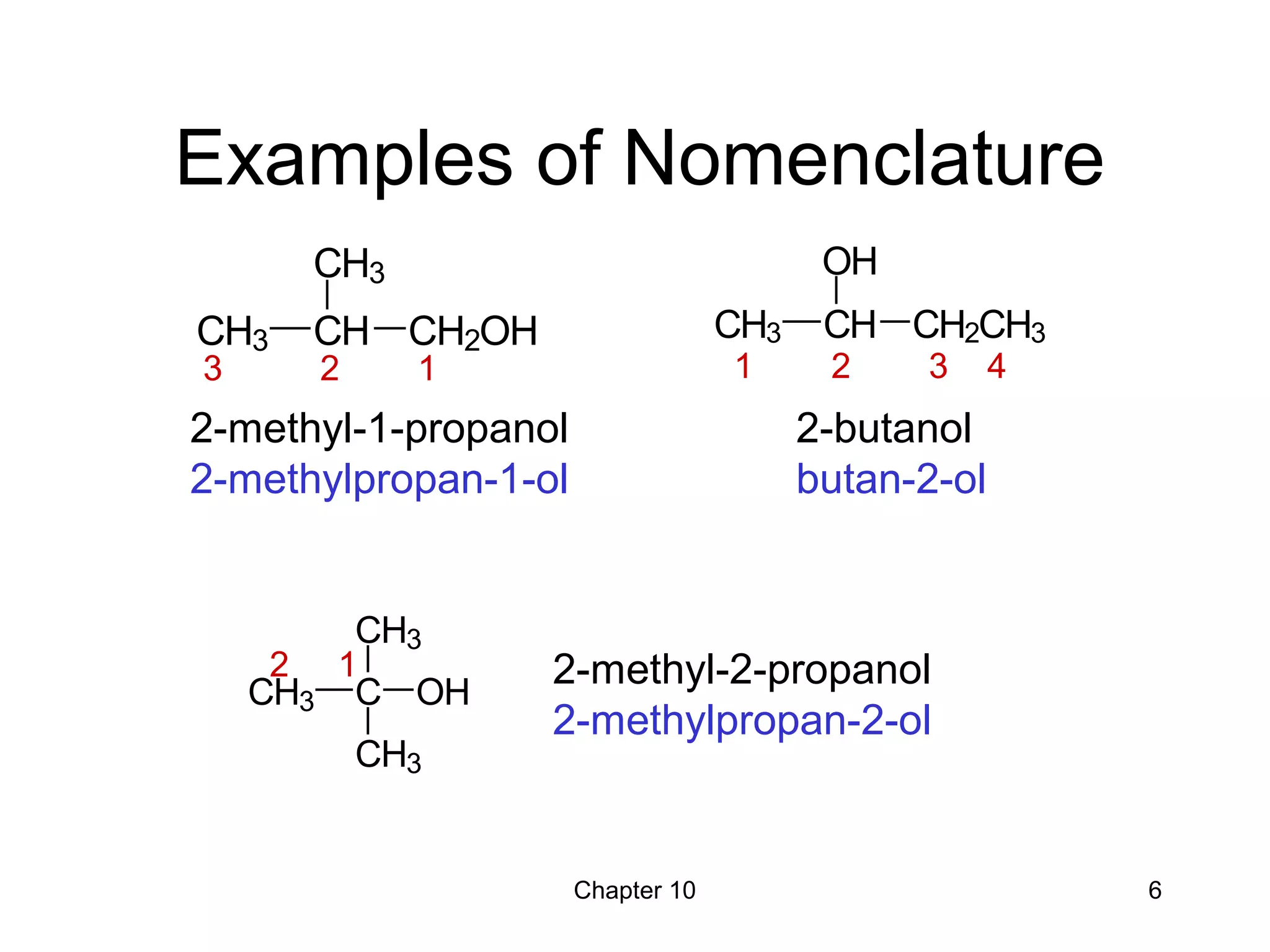
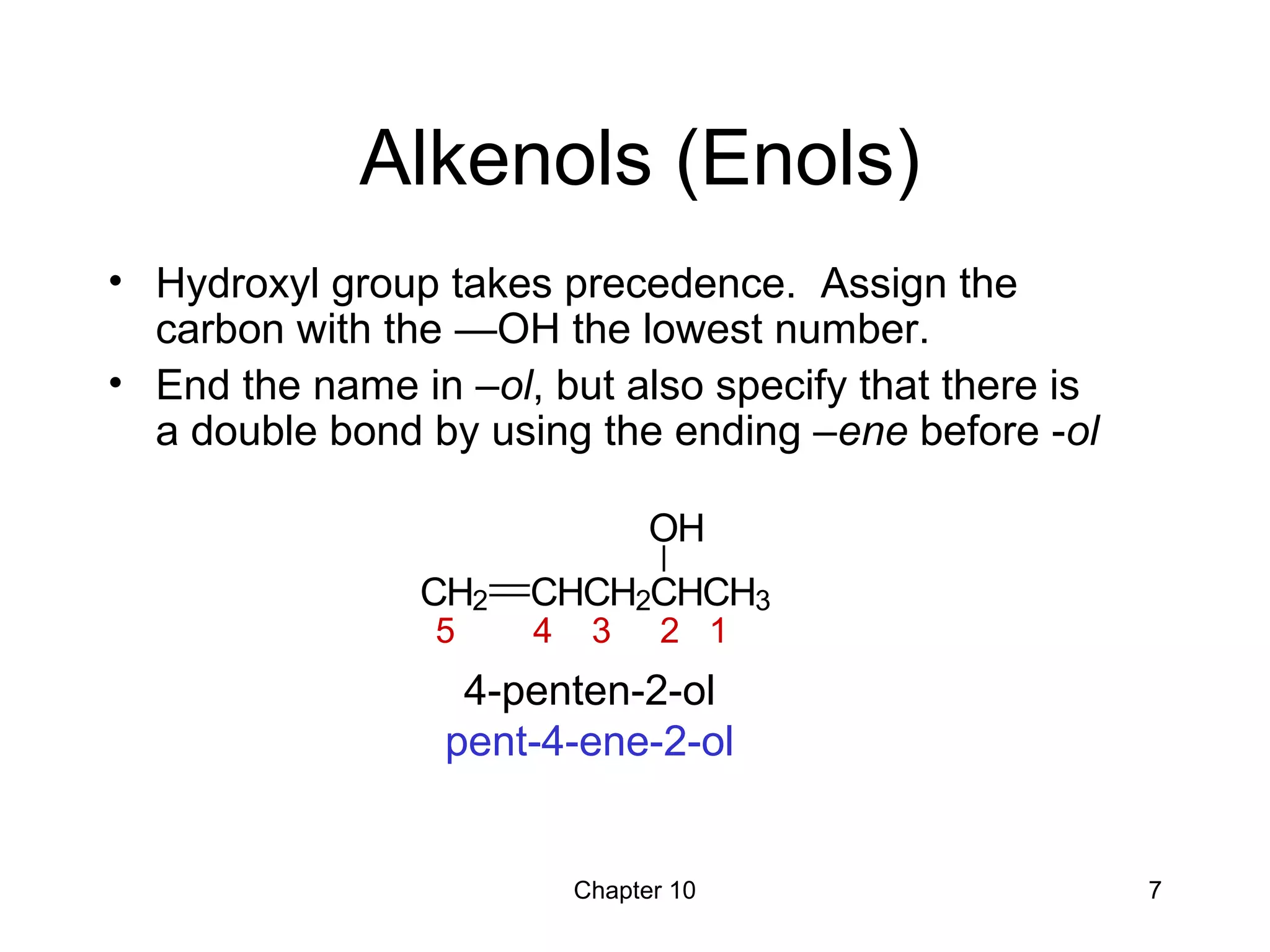
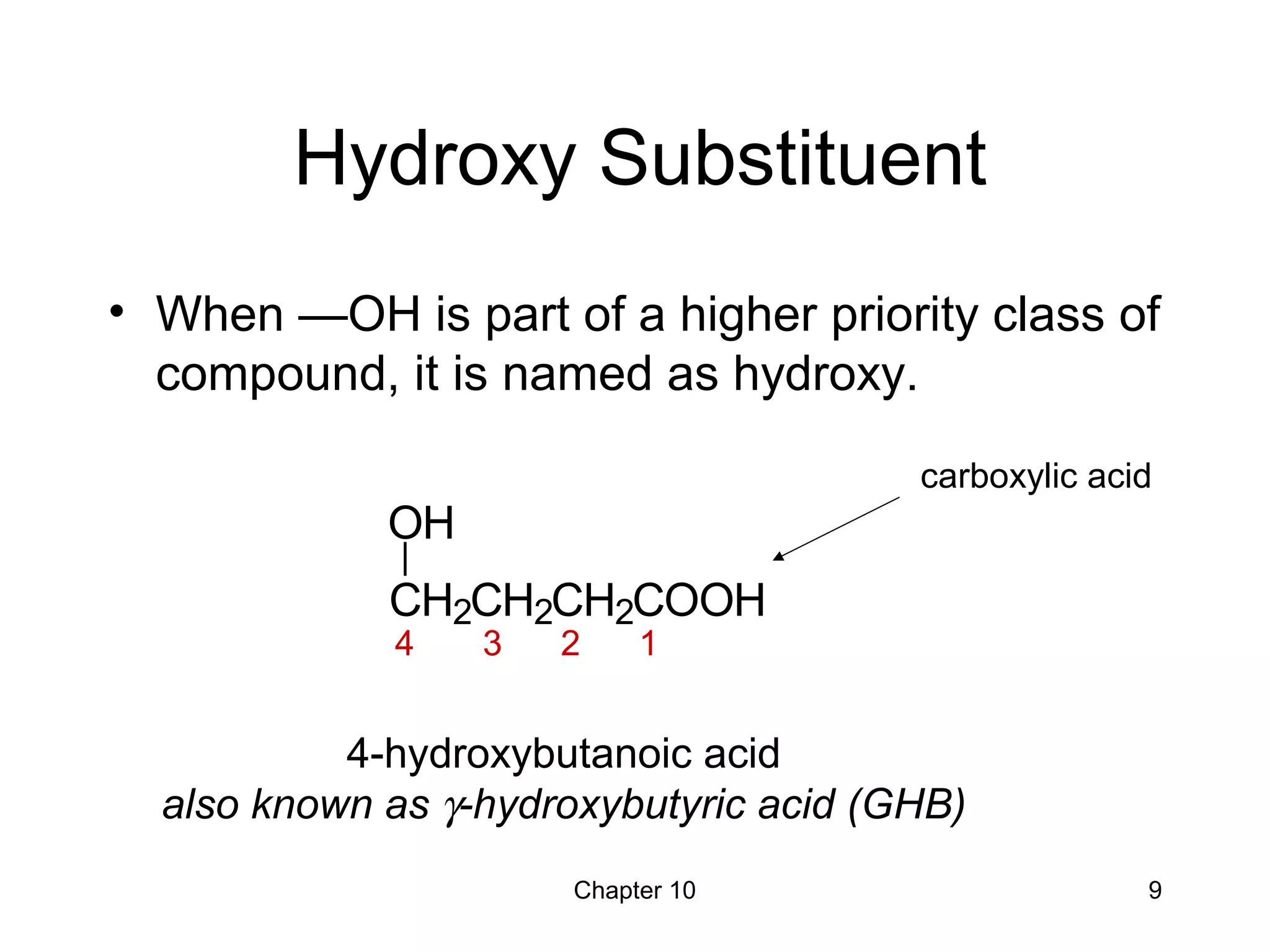
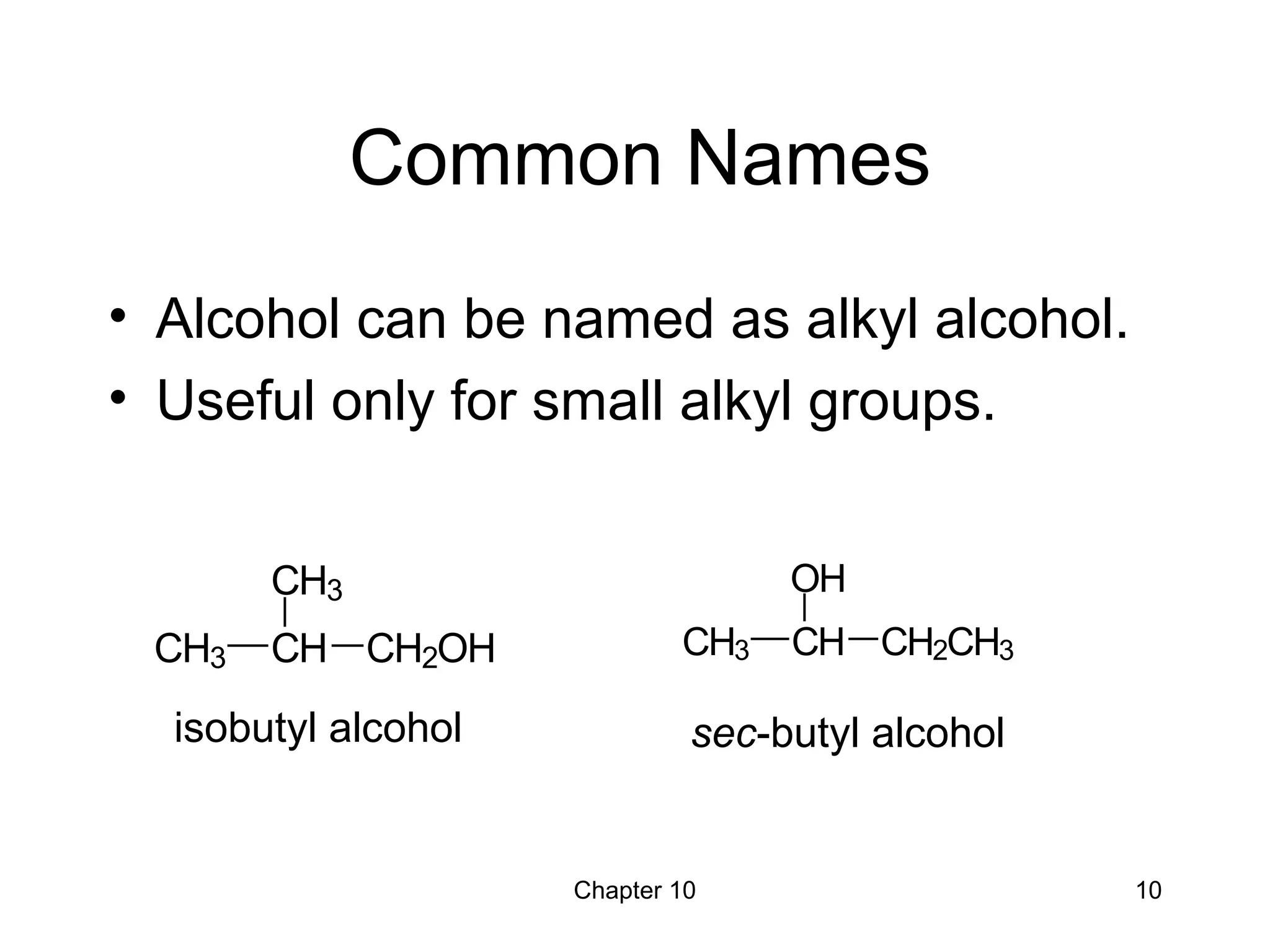
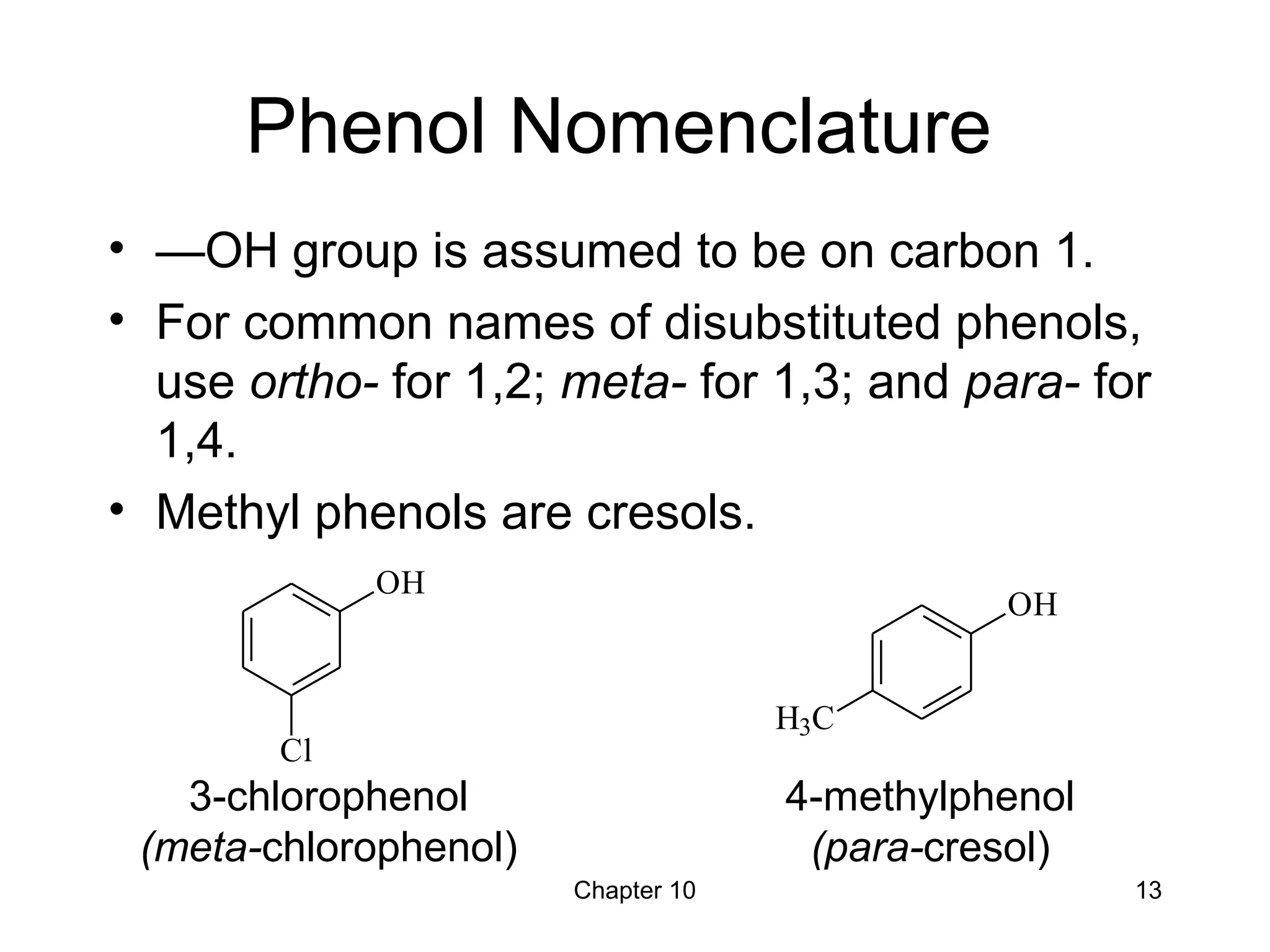
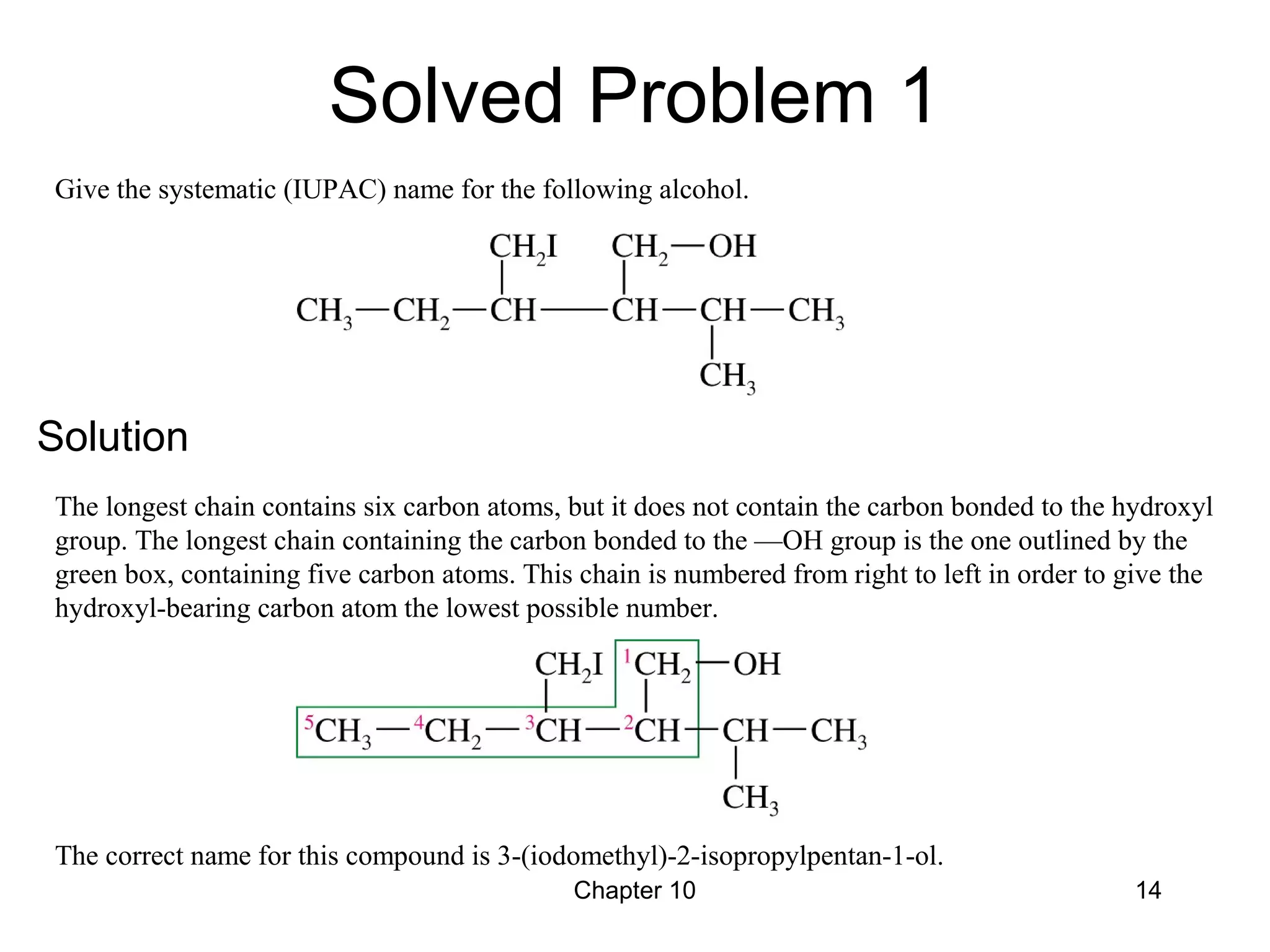
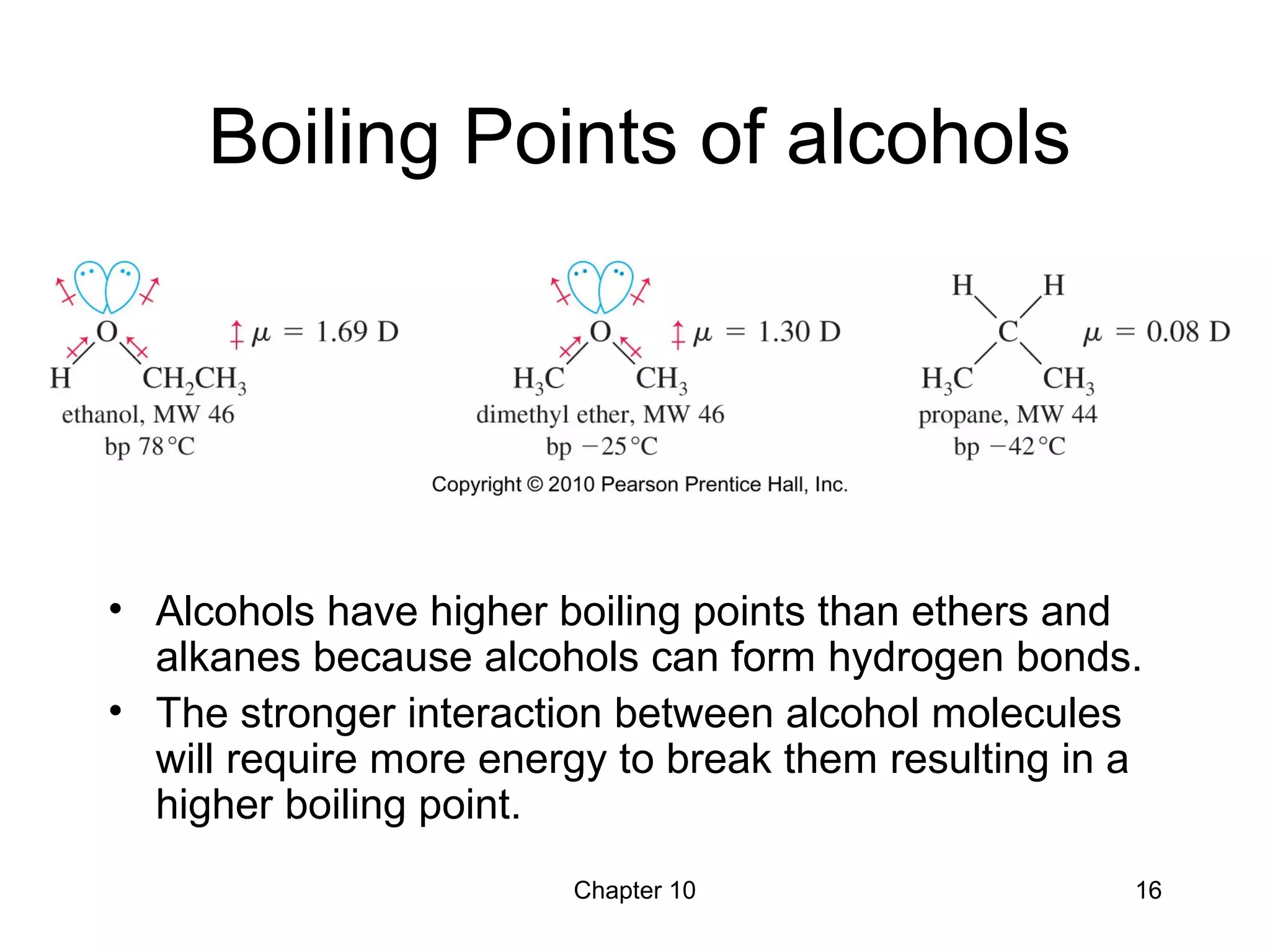
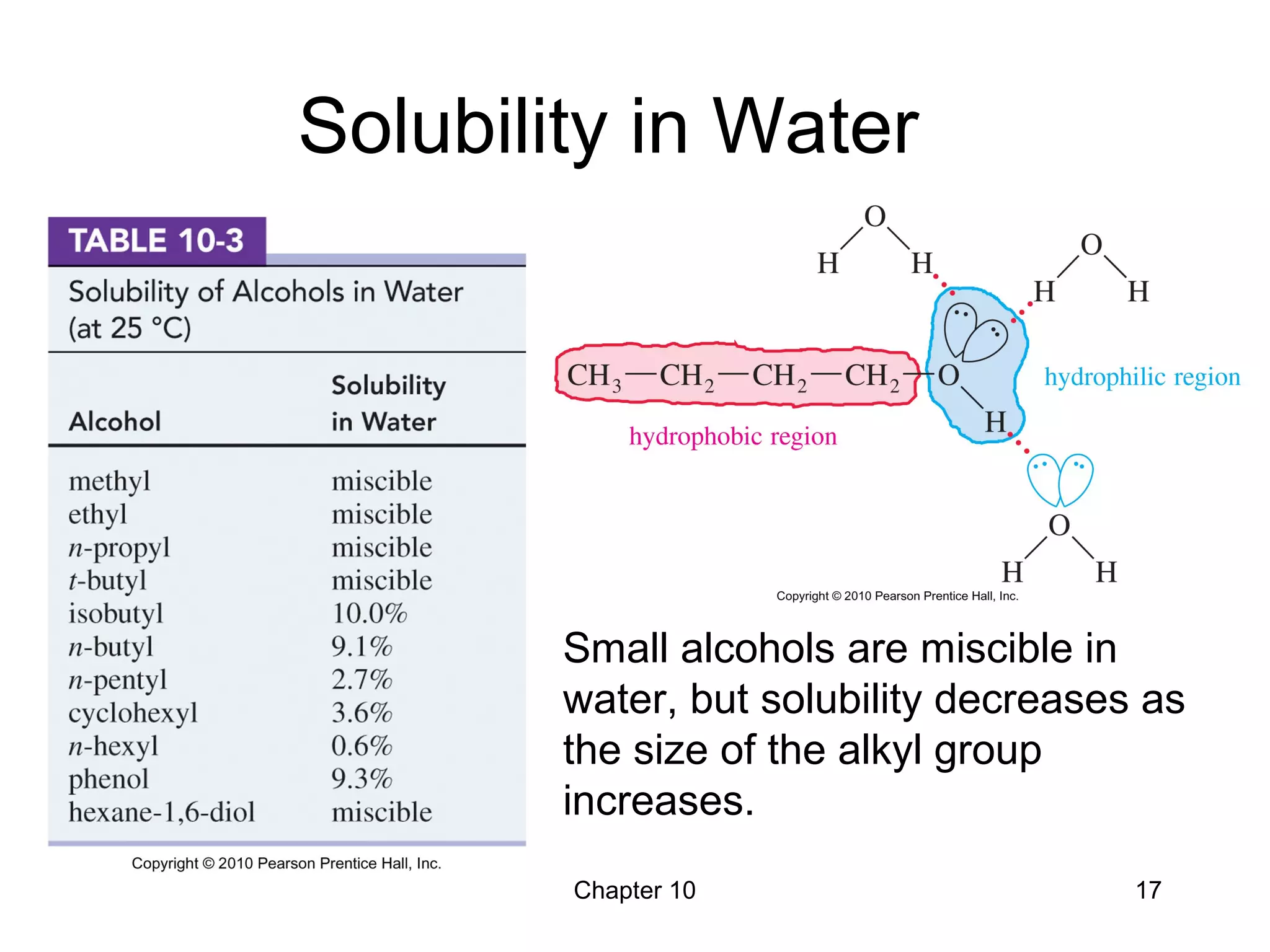
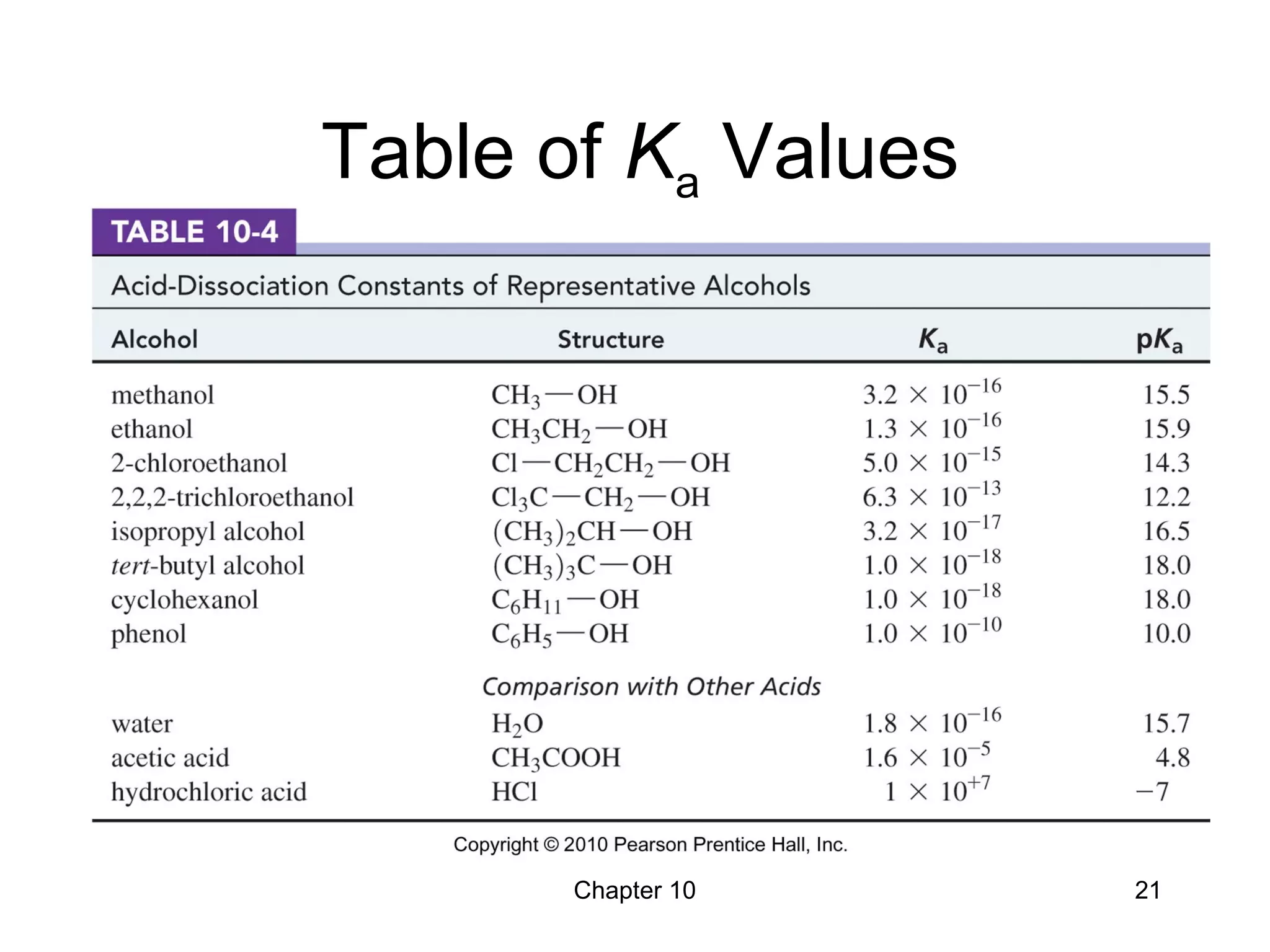
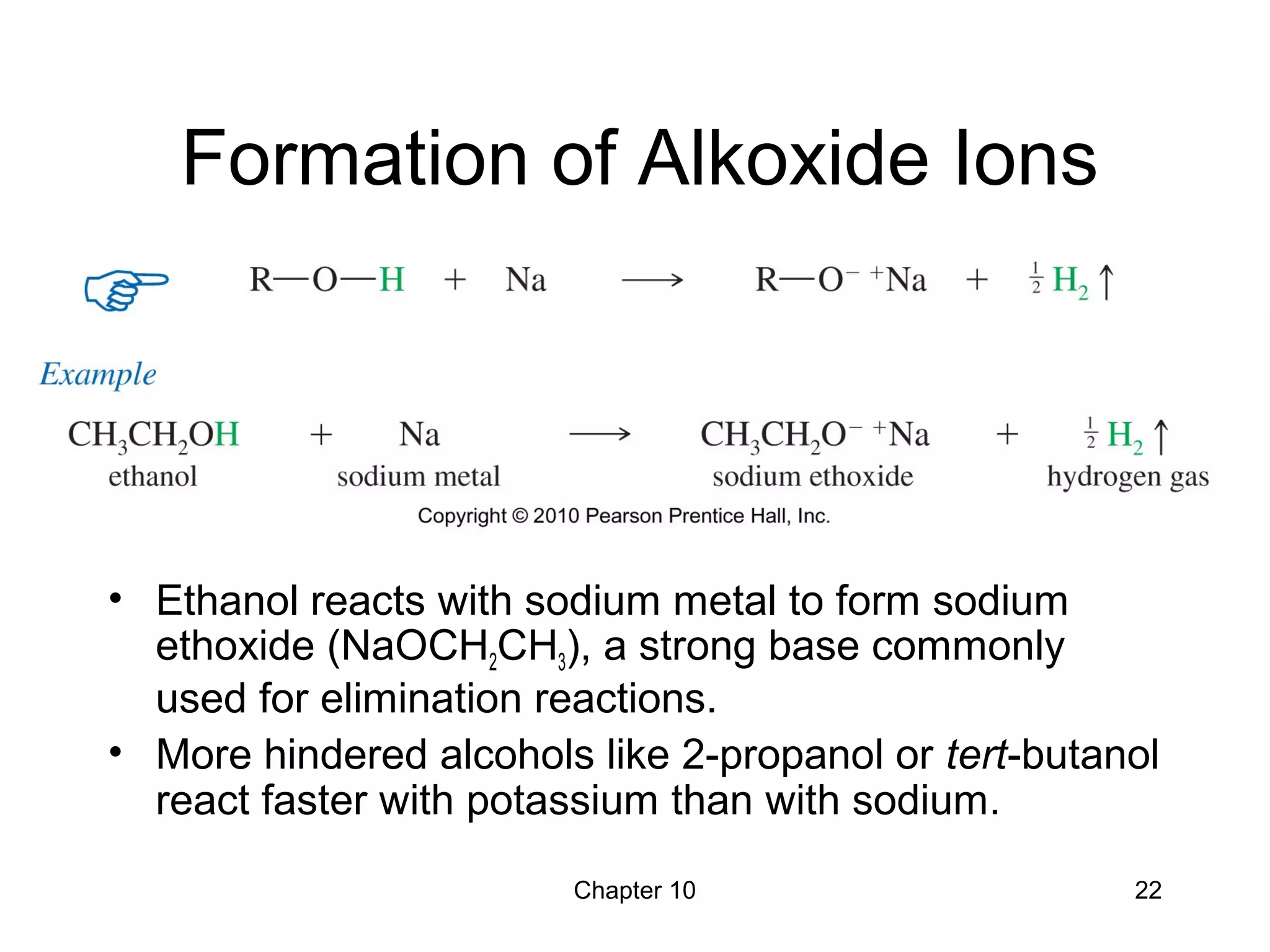
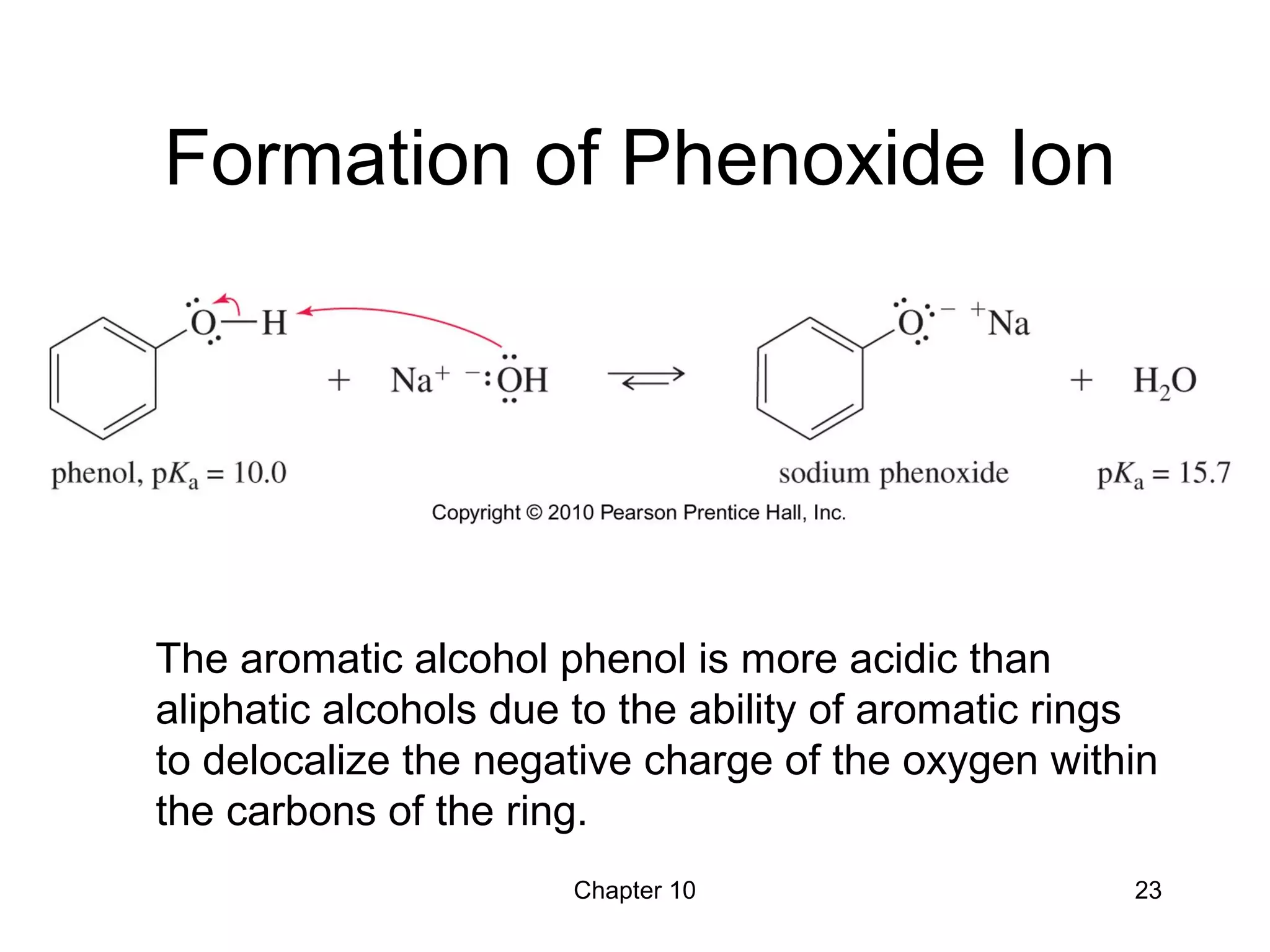
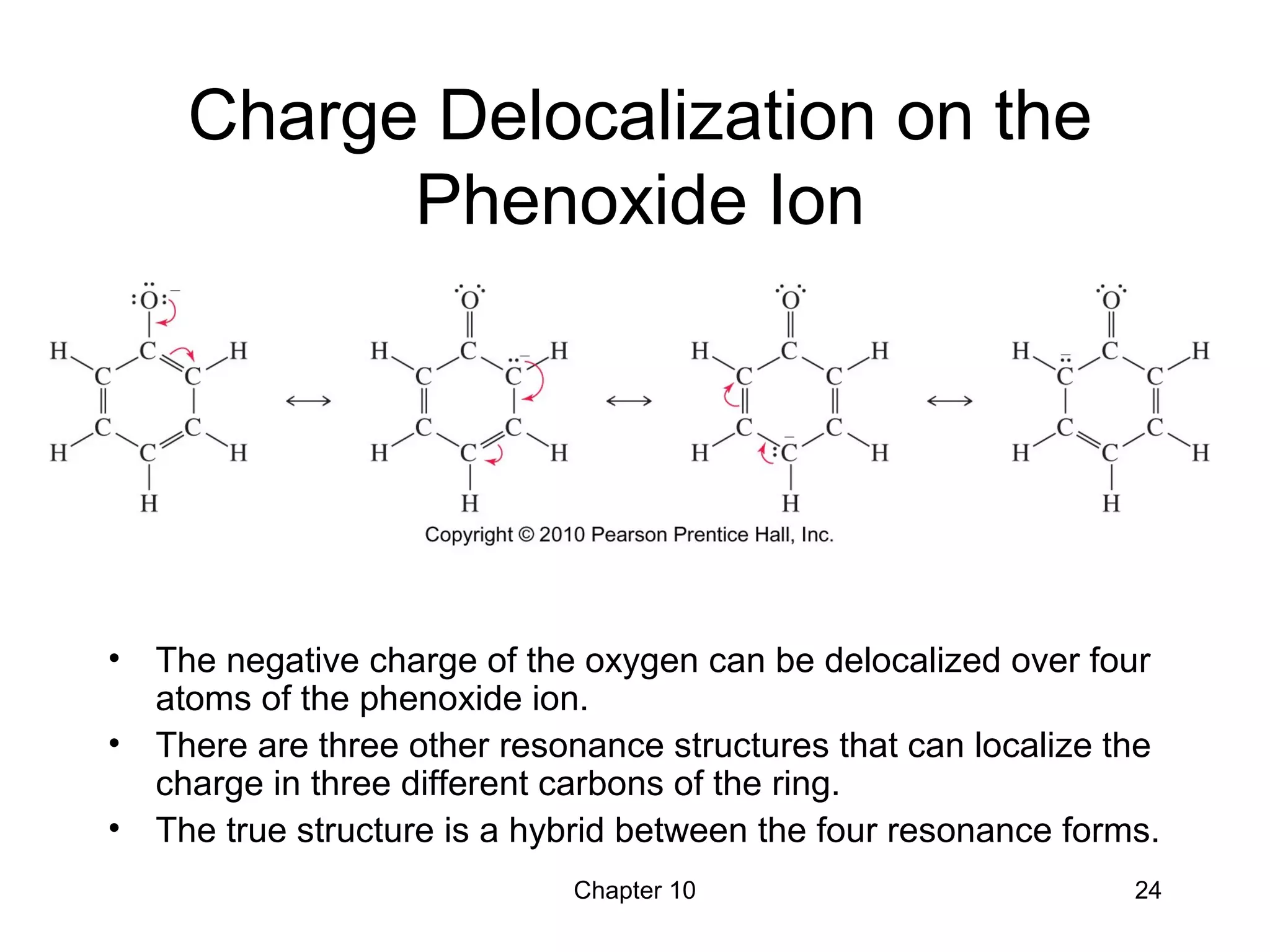
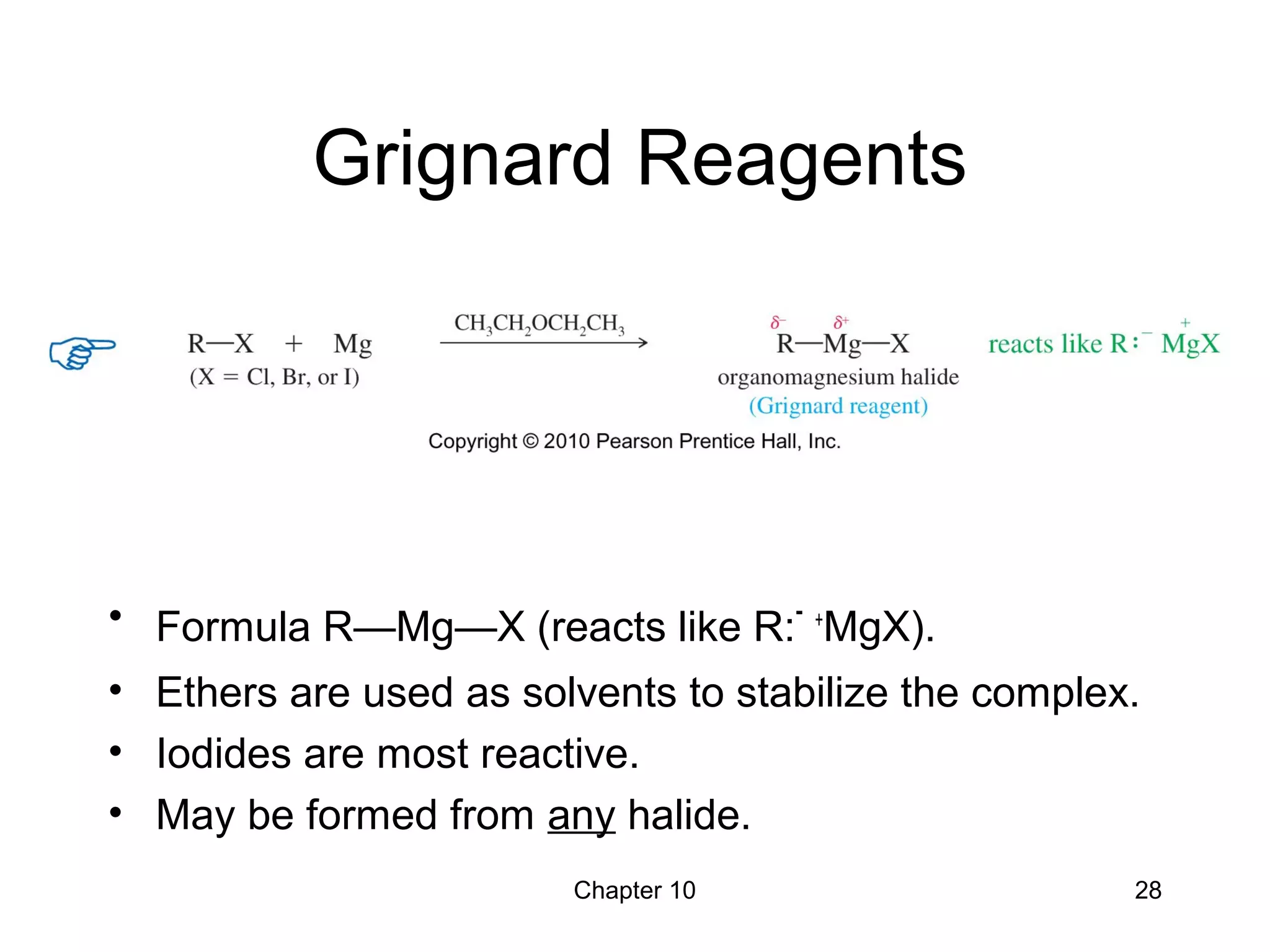
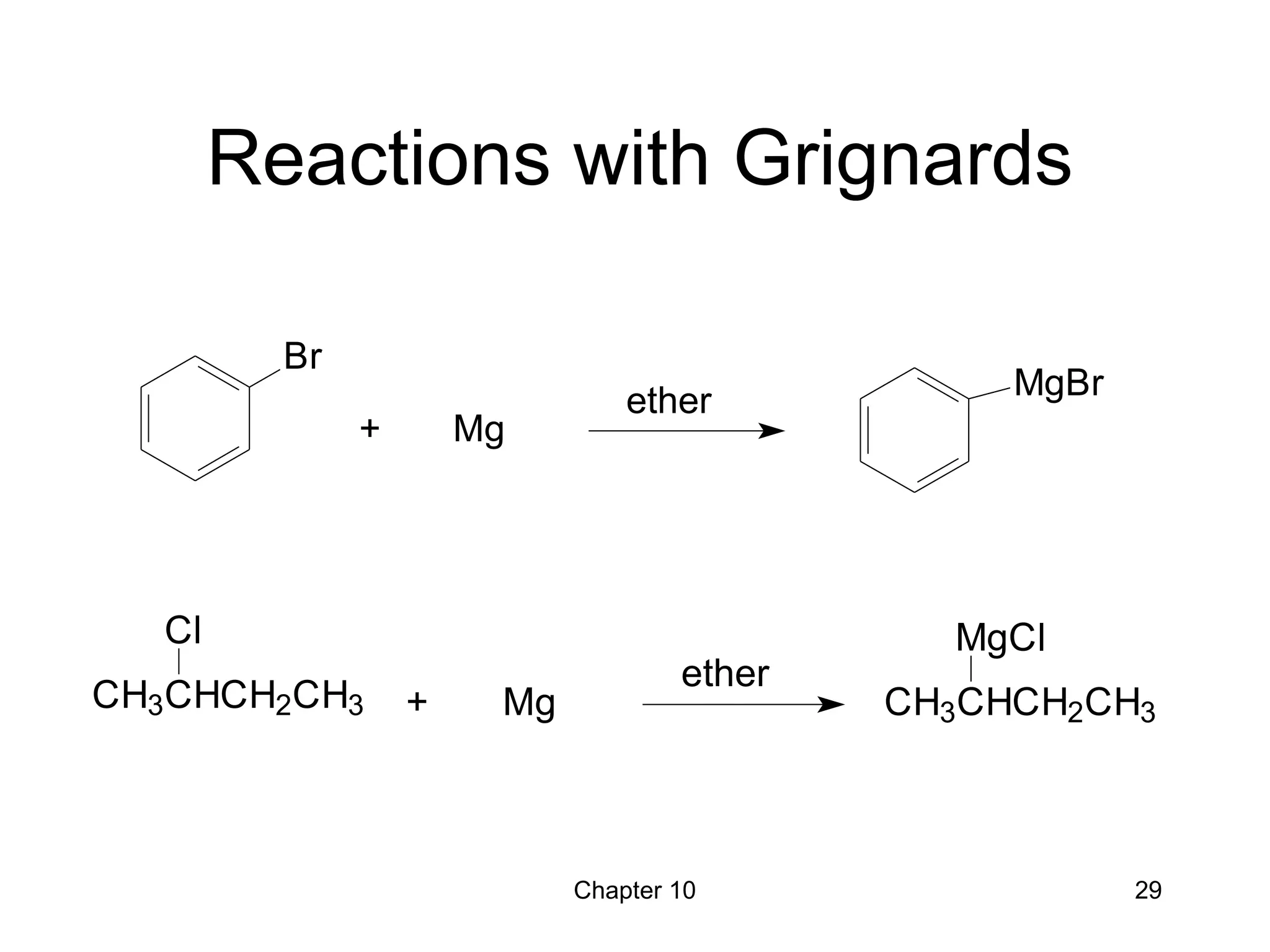
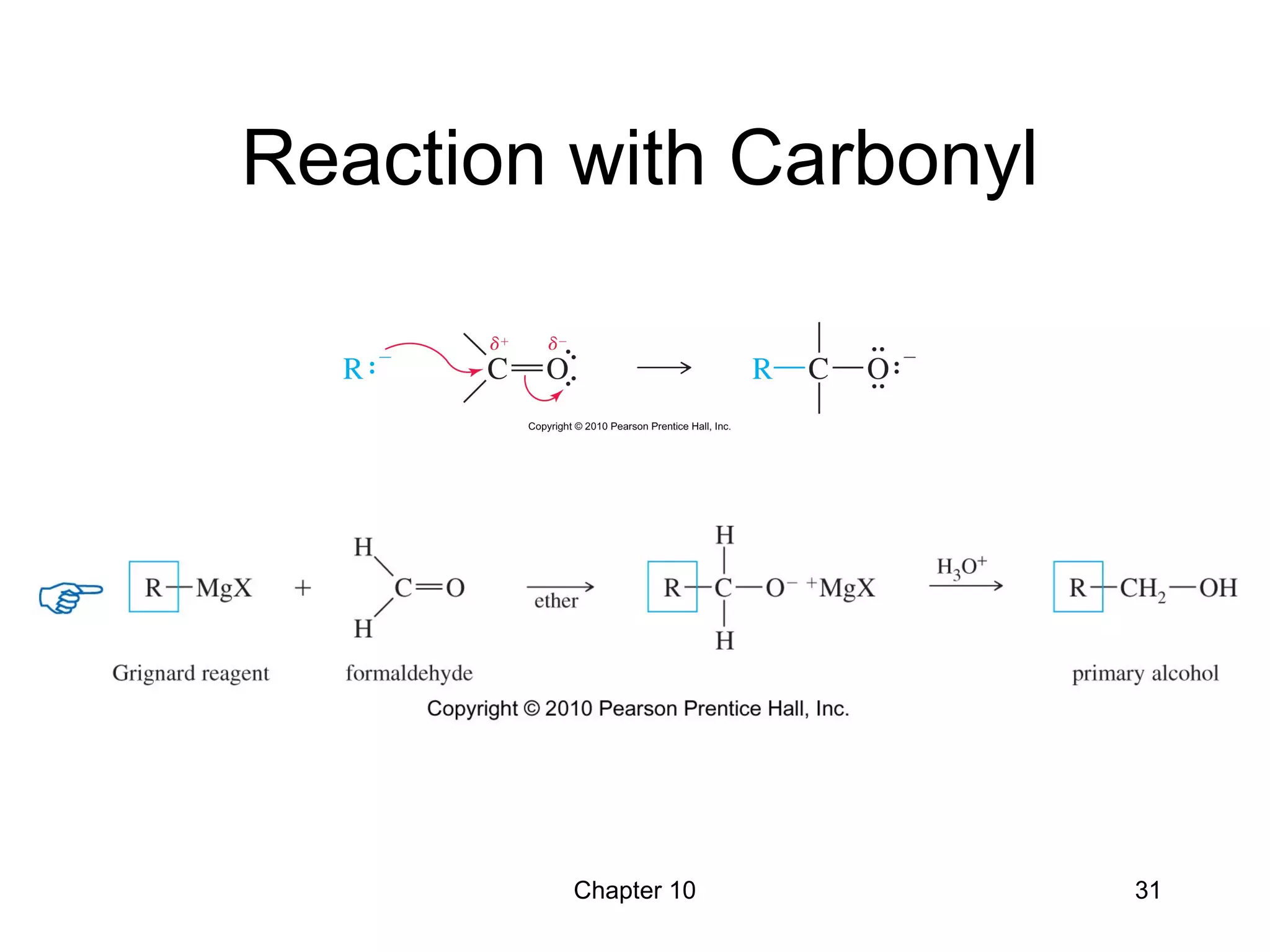
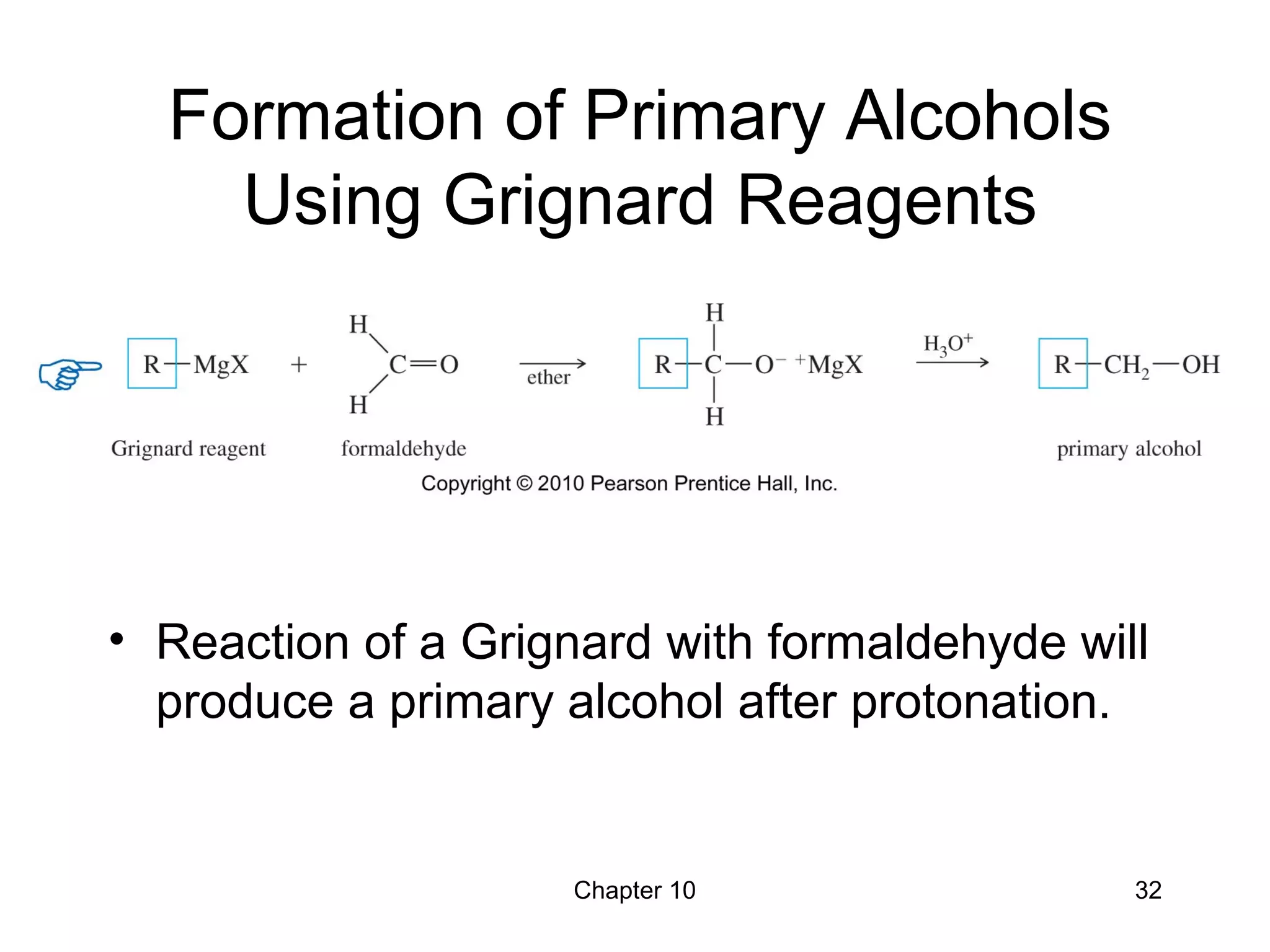
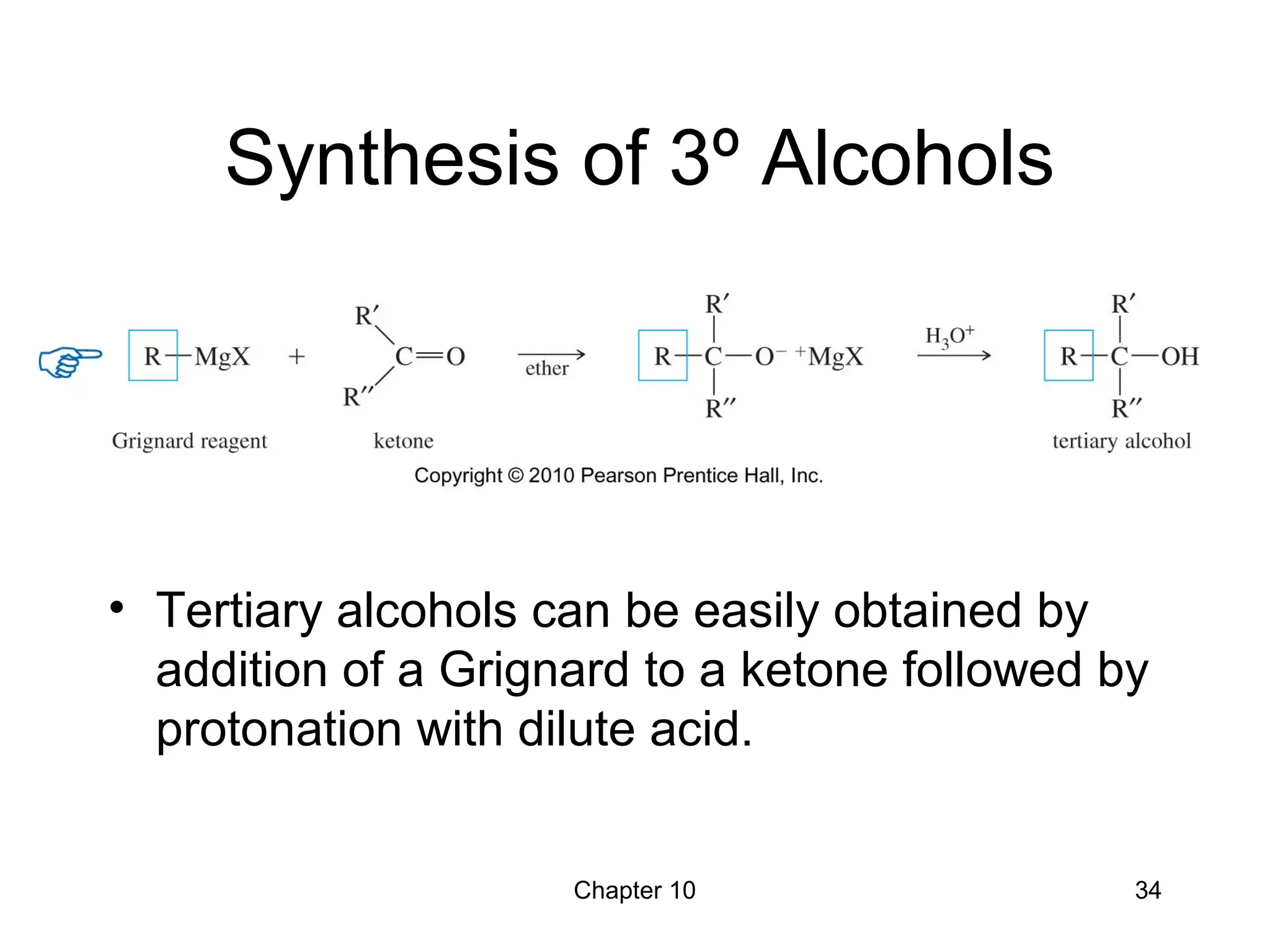
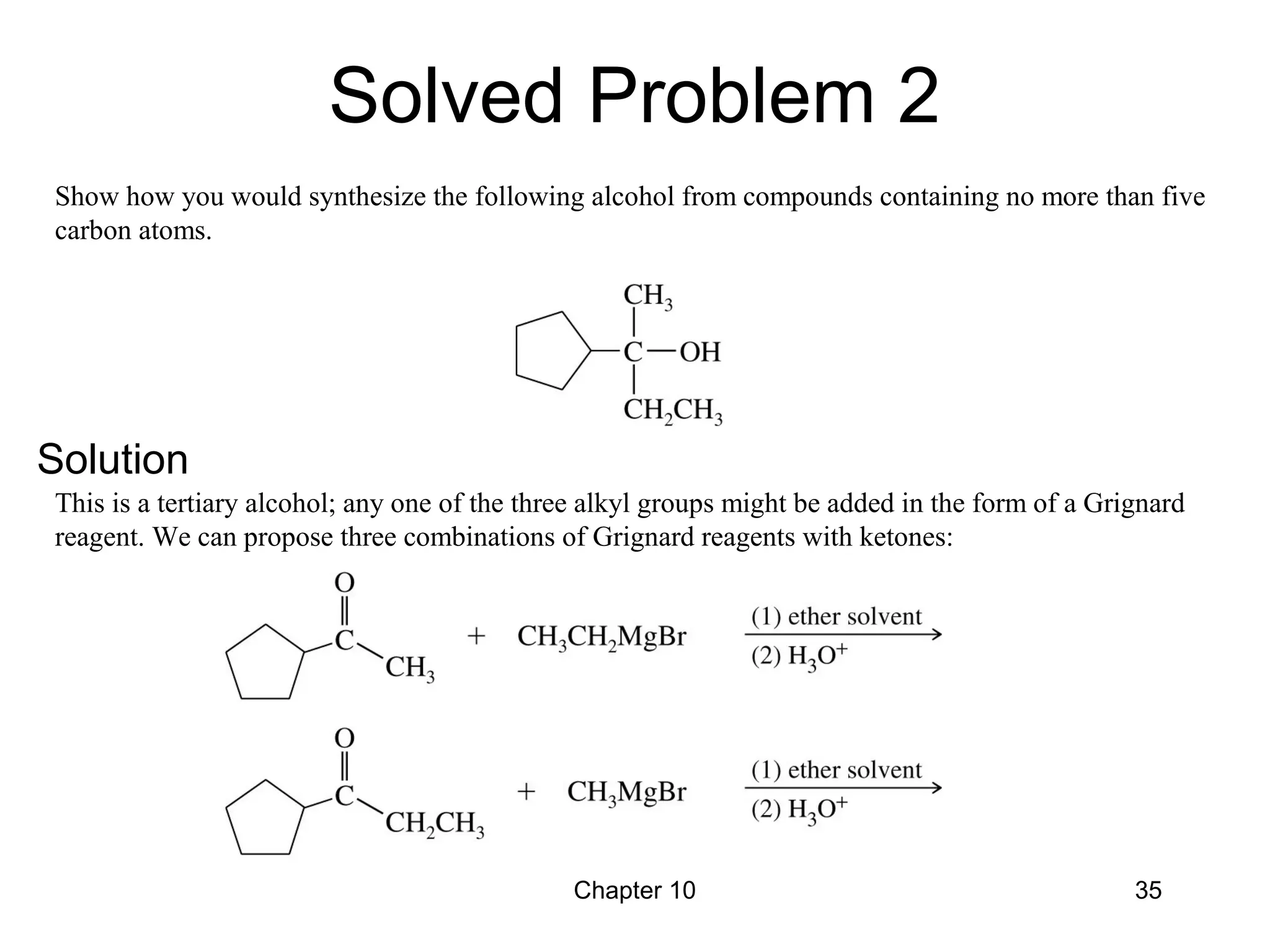
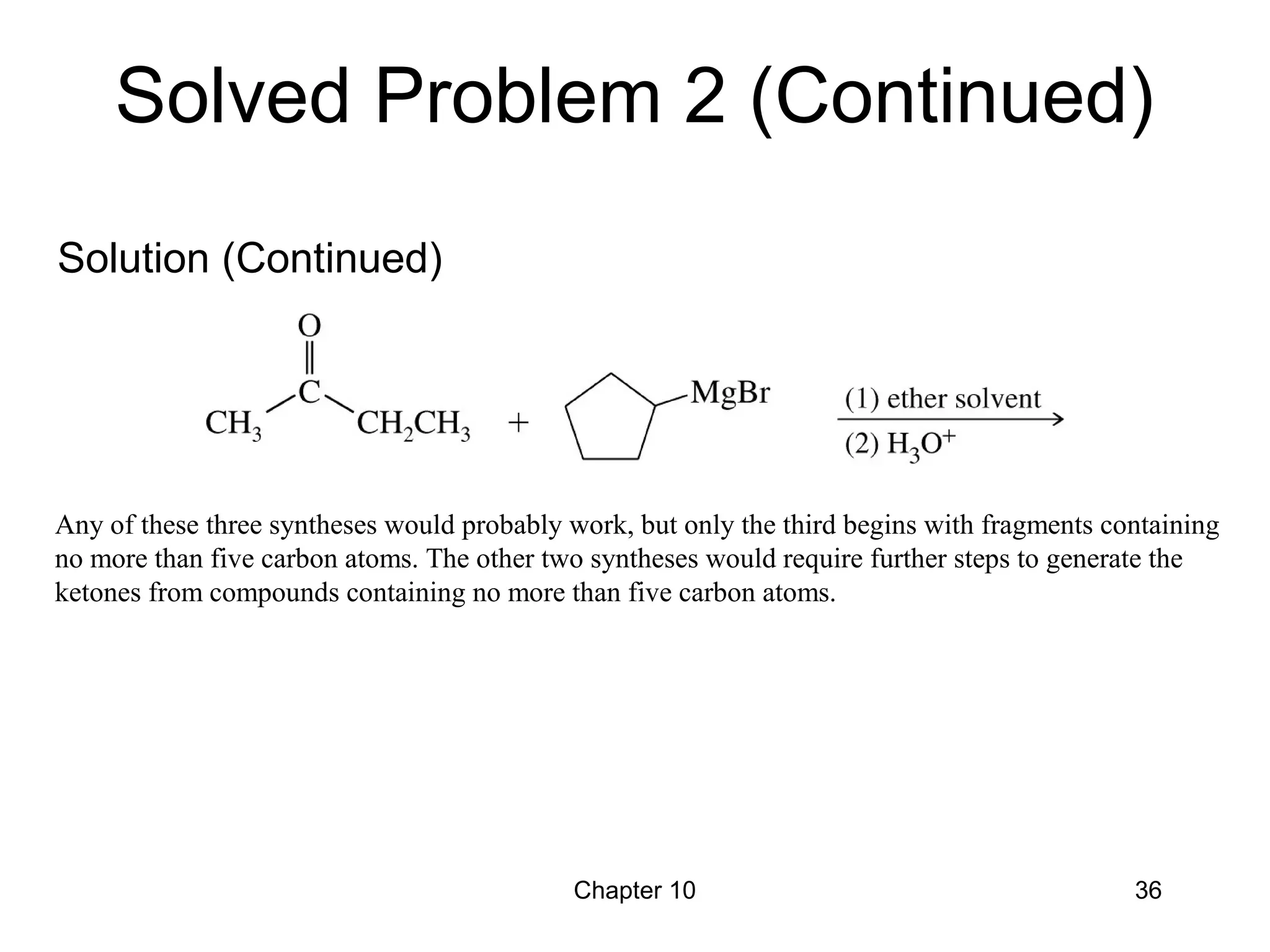
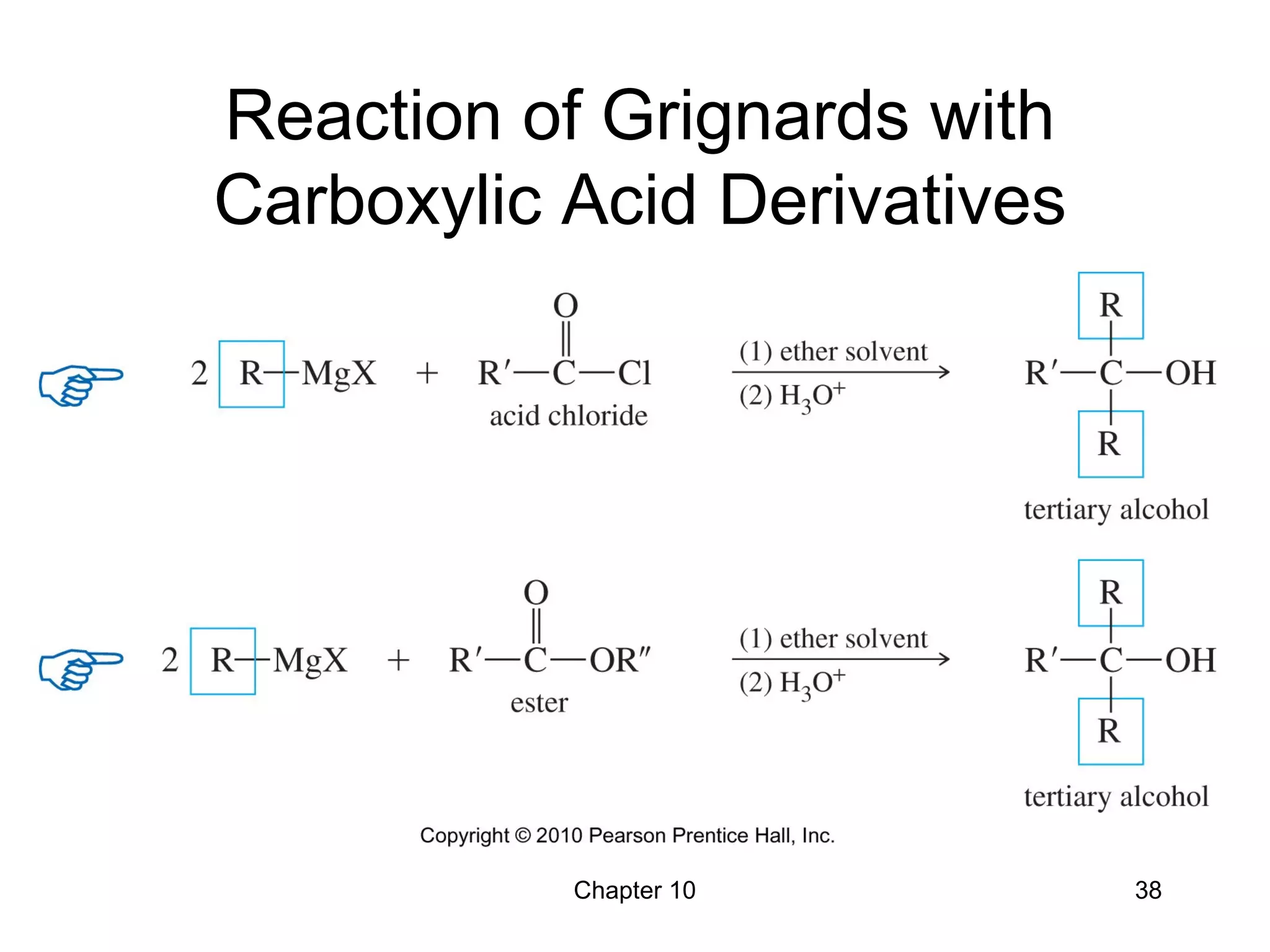
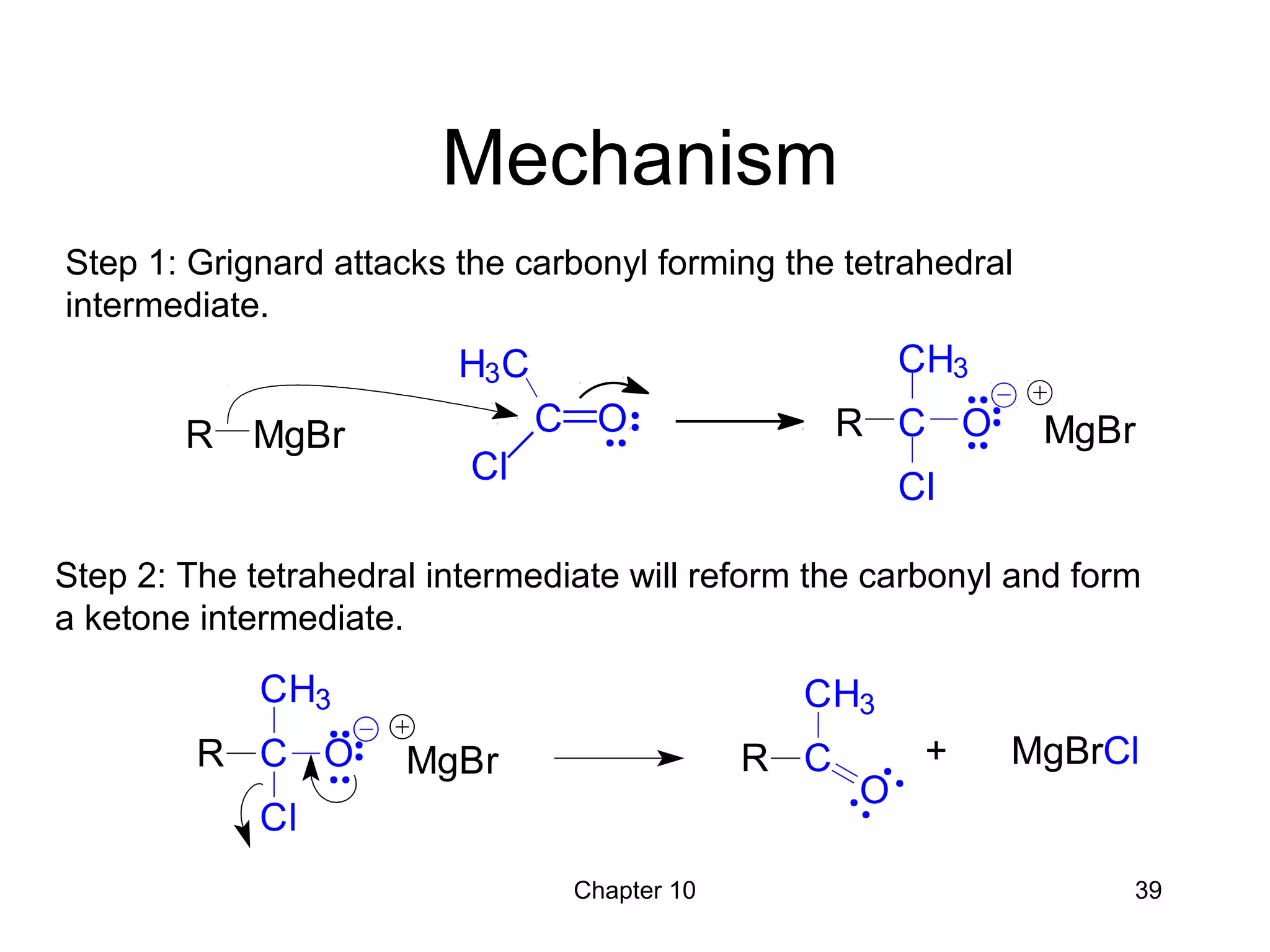
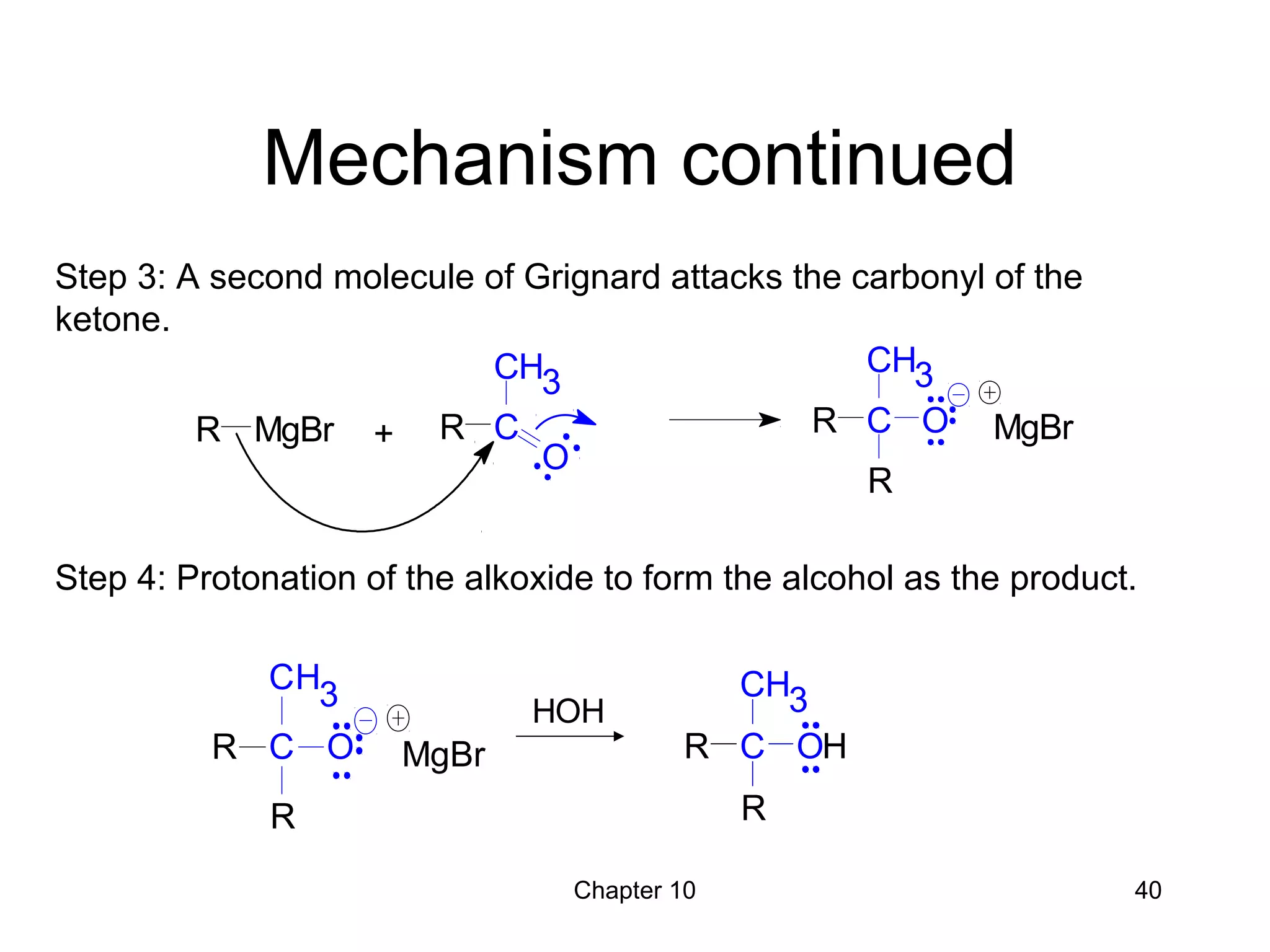
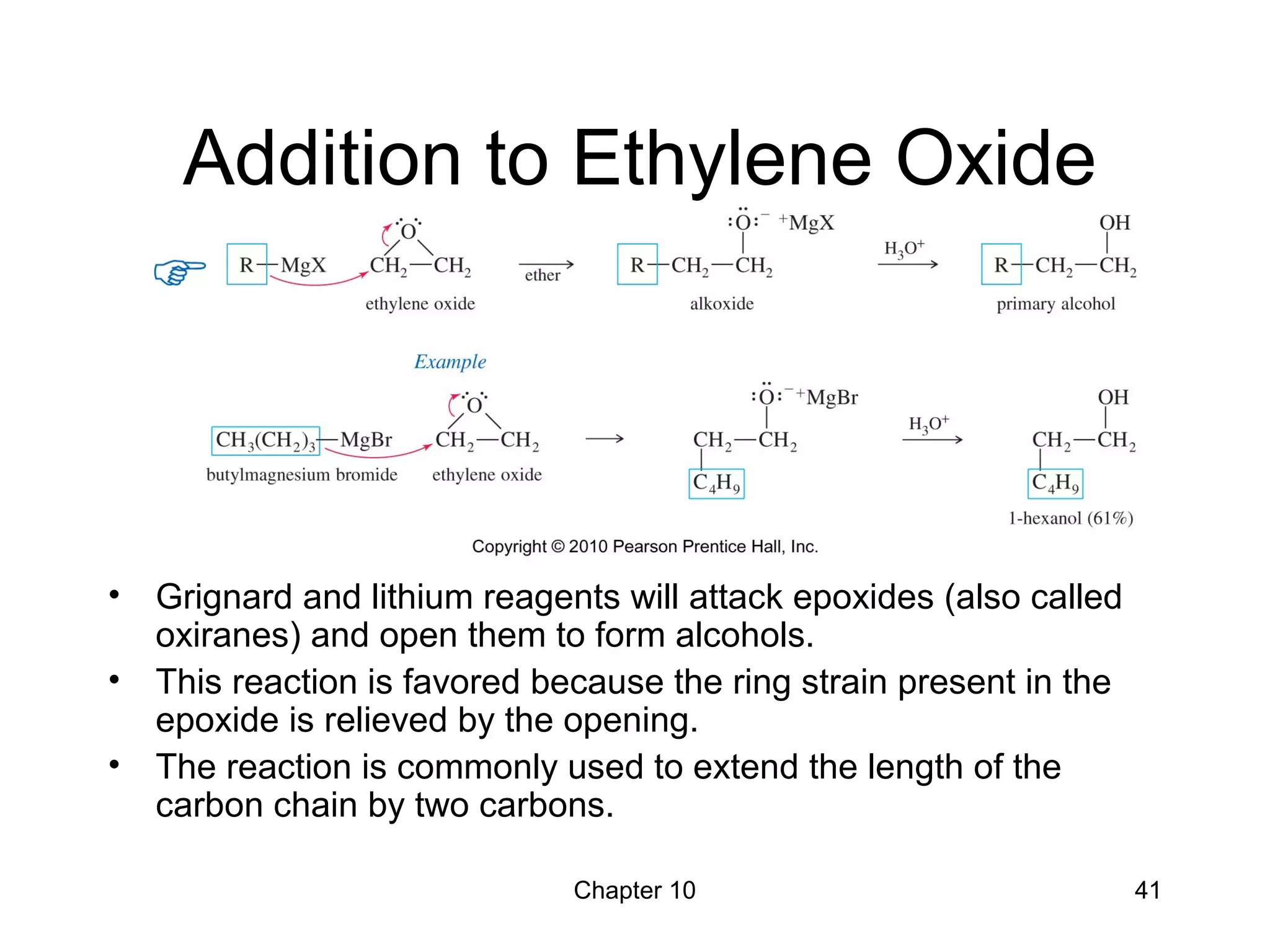
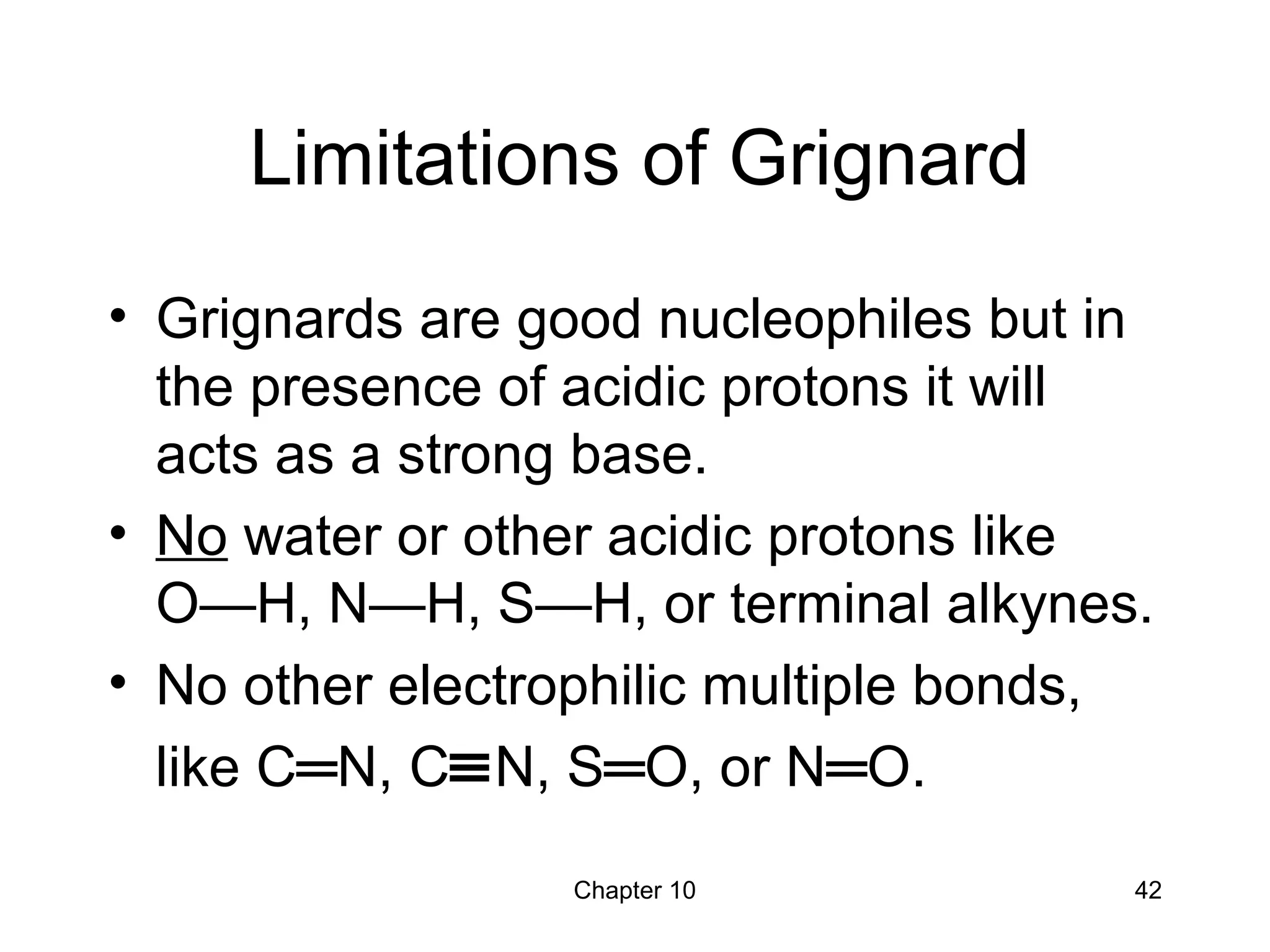
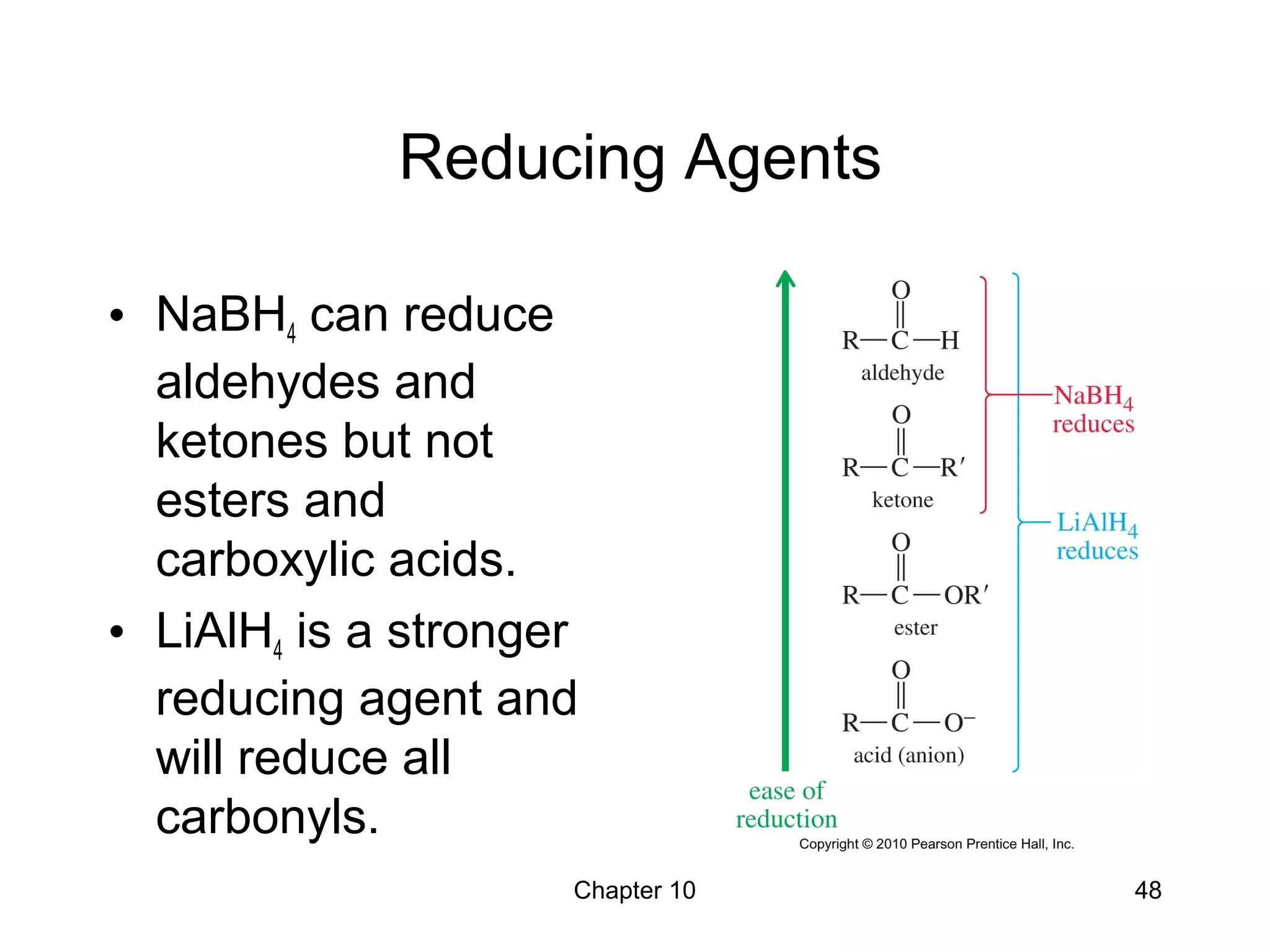
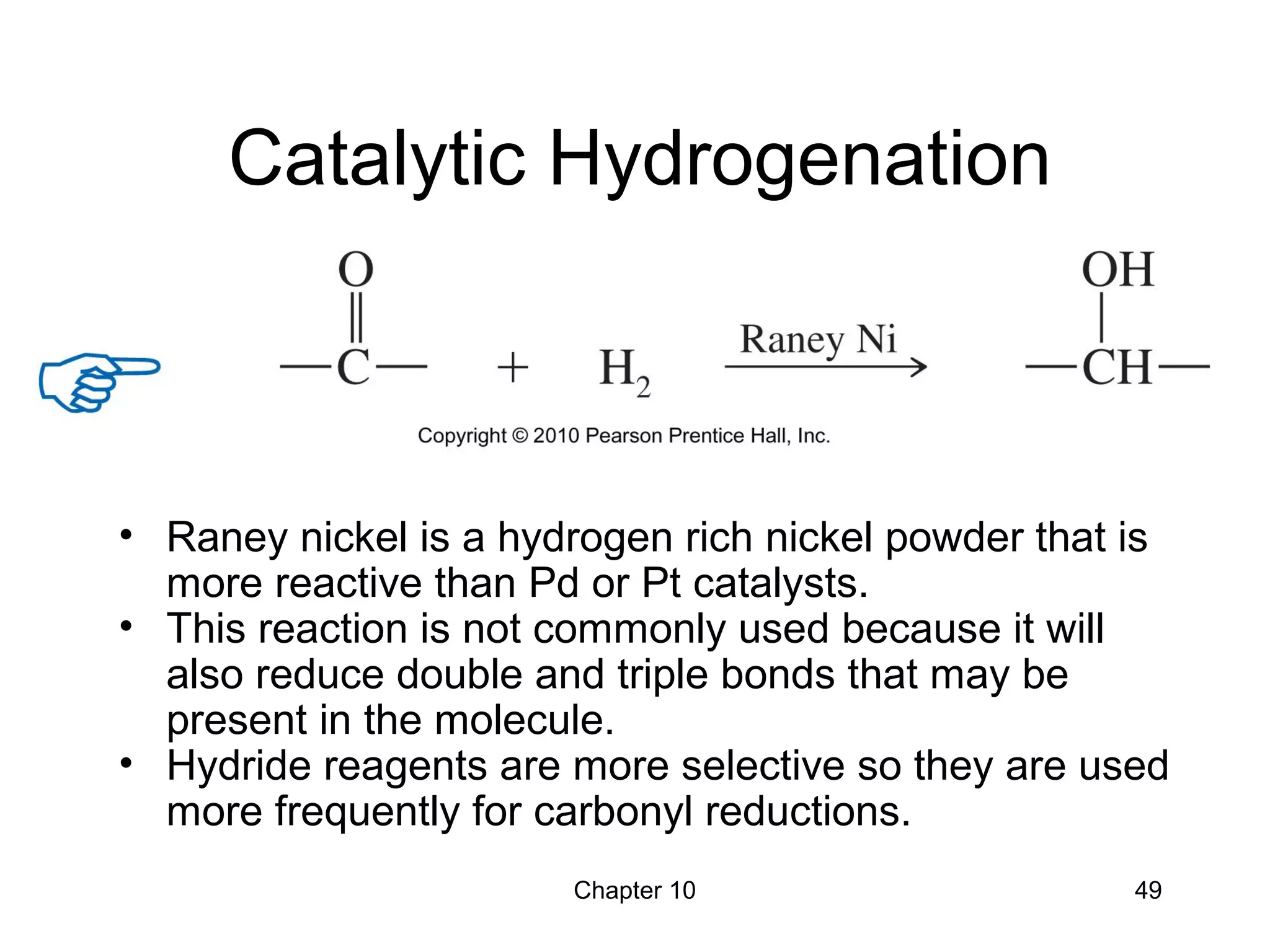
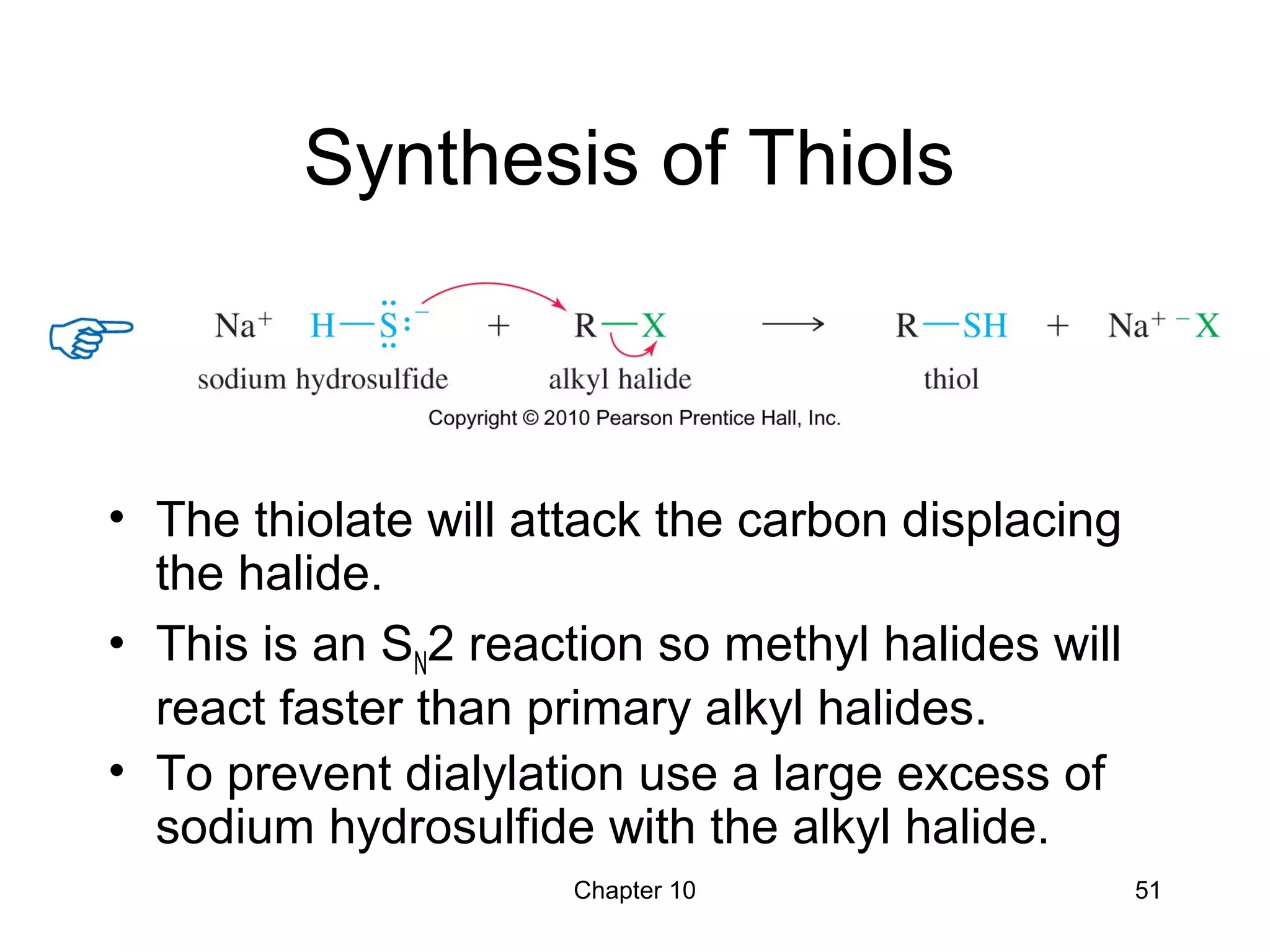
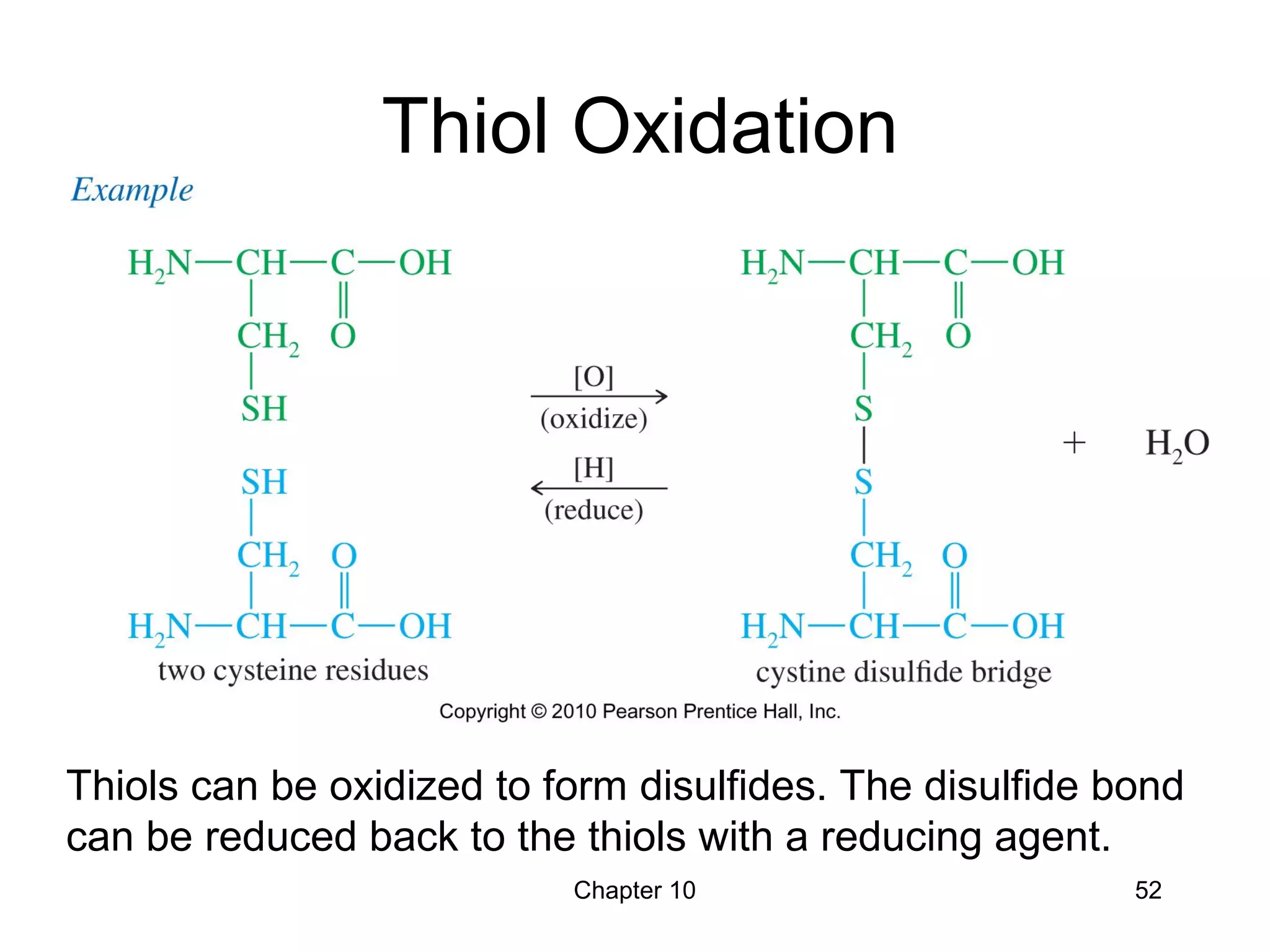
This document summarizes key concepts from Chapter 10 of Organic Chemistry regarding alcohols. It discusses the structure of alcohols and water, classification of primary, secondary, and tertiary alcohols. It also covers IUPAC nomenclature for naming alcohols, and common names. Physical properties like boiling points and solubility in water are described. Methods for synthesizing alcohols are summarized, including hydration of alkenes, use of organometallic reagents like Grignard reagents and organolithium reagents, and reduction of carbonyl groups.