Geo Chemisty
- 2. Introduction to Geochemistry The field of geochemistry involves: 1.The Study of the chemical composition of the Earth and other planets. 2.The Chemical processes and reactions that govern the composition of rocks, water, and soils. 3.The cycles of matter and energy that transport the Earth's chemical components in time and space, and their interaction with the hydrosphere and the atmosphere.
- 3. Introduction to Geochemistry Some subsets of geochemistry are: a)Isotope geochemistry: Determination of the relative and absolute concentrations of the elements and their isotopes in the earth and on earth's surface. b)Examination of the distribution and movements of elements in different parts of the earth (crust, mantle, hydrosphere etc.) and in minerals with the goal to determine the underlying system of distribution and movement. c)Cosmo chemistry: Analysis of the distribution of elements and their isotopes in the cosmos. d)Biogeochemistry: Field of study focusing on the effect of life on the chemistry of the earth. e)Organic geochemistry: A study of the role of processes and compounds that are derived from living or once-living organisms. f)Water Geochemistry: Understanding the role of various elements in watersheds. g)Regional, environmental and exploration geochemistry: Applications to environmental, hydrological and mineral exploration studies.
- 4. The main focus of geochemistry is to: Understand the principles governing the distribution and re- distribution of elements, ionic species and isotope ratios in earth materials, so that we can interpret the formation of mineral assemblages: conditions (P, T, etc.), processes (magmatic crystallization, weathering, chemical precipitation, metamorphism, etc.), and even the age. Predict changes in mineral assemblages (minerals, concentrations of elements, isotopic ratios) if a given mineral assemblage is subjected to different conditions (T, P, interaction with a fluid, etc.) Geochemistry plays an important role in forecasting the quality of crude oil in the accumulation. Geochemistry = chemistry of the Earth (i.e., of earth materials — minerals and rocks)
- 5. THE EARTH'S CHEMISTRY The bulk of the Earth is made from iron, oxygen, magnesium and silicon. More than 80 chemical elements occur naturally in the Earth and its atmosphere. Mostly Earth is composed of three parts: 1. Crust 2. Mantle (Upper & Lower) 3. Core The Earth's crust is a thin layer of rock that floats on the mantle. The crust is made mostly from oxygen and silicon (silicate minerals such as quartz), with aluminium, iron, calcium, magnesium, sodium, potassium, titanium and traces of 64 other elements. The upper mantle is made up of iron and magnesium silicates; the lower is silicon and magnesium sulphides and oxides. The core is mostly iron, with little nickel and traces of sulphur, carbon, oxygen and potassium.
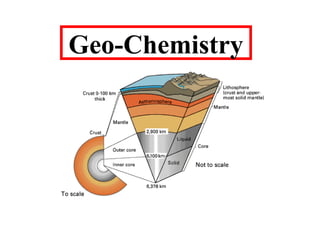
- 7. Fig.- This diagram shows the percentages of the chemical elements that make up the Earth. Fig.- This diagram shows the Earth interior.
- 8. EARTH'S INTERIOR The Earth's crust is a thin hard outer shell of rock. Under the crust, there is a deep layer of hot soft rock called the mantle. The crust and upper mantle can be divided into three layers according to their rigidity: 1.The lithosphere (The lithosphere is the upper, rigid layer of the Earth. It consists of the crust and the top of the mantle and it is about 100 km thick). 2.The asthenosphere (Below the lithosphere, in the Earth's mantle, is the hot, soft rock of the asthenosphere. The boundary between the lithosphere and the asthenosphere occurs at the point where temperatures climb above 1300°C). 3. The mesosphere. the solid part of the earth's mantle lying between the asthenosphere and the core.
- 9. EARTH'S INTERIOR Beneath the mantle is a core of hot iron and nickel. The outer core is so hot (4500°C - 6000°C) that it is always molten. The inner core is even hotter (up to 7000°C) but it stays solid because the pressure is 6000 times greater than on the surface. The inner core contains 1.7% of the Earth's mass, the outer core 30.8%; the core - mantle boundary 3%; the lower mantle 49%; the upper mantle 15%; the ocean crust 0.099% and the continental crust 0.374%.
- 10. Fig.- The main layers that form the Earth. Our knowledge of the Earth's interior comes mainly from studying how earthquake waves move through different kinds of rock. Analysis of how earthquake waves are deflected reveals where different materials occur in the interior. S (secondary) waves pass only through the mantle. P (primary) waves pass through the core as well. P waves passing through the core are deflected, leaving a shadow zone where no waves reach the far side of the earth. The speed of earthquake waves reveals how dense the rocky materials are. Cold, hard rock transmits waves more quickly than hot, soft rock.
- 11. Geo Chemical Classification of Elements There are several trials to classify elements on geochemical basis. Names such as siderophile, chalcophile, lithophile, hydrophile, thalassophile, atmophile are commonly used to denote the particular geochemical affinity of elements. chemical affinity is the electronic property by which dissimilar chemical species are capable of forming chemical compounds or Affinity is the tendency of a molecule to associate with another. . Chemical affinity can also refer to the tendency of an atom or compound to combine by chemical reaction with atoms or compounds of unlike composition.
- 12. Geochemical Affinity In the classification scheme of Goldschmidt, elements are divided according to how they partition between coexisting silicate liquid, sulfide liquid, metallic liquid, and gas phase… Silicate Liquid Sulfide Liquid Metallic Liquid Gas PhaseGas Phase Siderophile Chalcophile Lithophile Atmophile H, He, N, Noble gases Alkalis, Alkaline Earths, Halogens, B, O, Al, Si, Sc, Ti, V, Cr, Mn, Y, Zr, Nb, Lanthanides, Hf, Ta, Th, U Cu, Zn, Ga, Ag, Cd, In, Hg, Tl, As, S, Sb, Se, Pb, Bi, Te Fe, Co, Ni, Ru, Rh, Pd, Os, Ir, Pt, Mo, Re, Au, C, P, Ge, Sn Melting a chondrite gives 3 immiscible liquids plus vapor:
- 13. H Li Na K Rb Cs Fr Be Mg Ca Sr Ba Ra Sc Y Ti Zr Hf Rf V Nb Ta Db Cr Mo W Sg Mn Tc Re Bh Fe Ru Os Hs Co Rh Ir Mt Ni Pd Pt Cu Ag Au Zn Cd Hg Ga In Tl Ge Sn Pb As Sb Bi Se Te Po Br I At Kr Xe Rn B Al C Si N P O S F Cl Ne Ar He La Ac Ce Th Pr Pa Nd U Pm Np Sm Pu Eu Am Gd Cm Tb Bk Dy Cf Ho Es Er Fm Tm Md Yb No Lu Lr 1 2 3 4 5 6 7 IA IIA IIIA IVA VA VIA VIIA VIIIA IIIB IVB VB VIB VIIB VIIIB IB IIB Lanthanides Actinides 1 3 11 19 37 55 87 4 12 20 38 56 88 21 39 22 40 72 104 23 41 73 105 24 42 74 106 25 43 75 107 26 44 76 108 27 45 77 109 28 46 78 29 47 79 30 48 80 5 13 31 49 81 6 14 32 50 82 7 15 33 51 83 8 16 34 52 84 9 17 35 53 85 10 18 36 54 86 2 57 89 58 90 59 91 60 92 61 93 62 94 63 95 64 96 65 97 66 98 67 99 68 100 69 101 70 102 71 103 Atmophile Lithophile Chalcophile Siderophile Artificial What makes an element siderophile or lithophile?What makes an element siderophile or lithophile? Notably, the Goldschmidt categories are well-Notably, the Goldschmidt categories are well- grouped in thegrouped in the periodic table of the elementsperiodic table of the elements::
- 14. Lithophile elements: Lithophile elements mainly consist of the highly reactive metals of the s-and f-blocks. They also include a small number of reactive nonmetals, and the more reactive metals of the d-block such as titanium, zirconium and vanadium. Siderophile elements: Siderophile elements are the high-density transition metals that tend to bond with metallic iron in the solid or molten state. Chalcophile elements: Chalcophile elements are those metals (sometimes called "poor metals") and heavier nonmetals that have a low affinity for oxygen and prefer to bond with sulfur as highly insoluble sulfides. Atmophile elements: Atmophile elements are defined as those that are found chiefly or exclusively in the form of gases.
- 15. Ionization potential Energy required to remove the least tightly bound electron. Electron affinity Energy given up as an electron is added to an element. Electronegativity Quantifies the tendency of an element to attract a shared electron when bonded to another element. Properties derived from outer electrons
- 18. Chemical Weathering Weathering is a process of the disintegration and degeneration of rocks minerals or soils as a result of direct contact with the atmosphere of the Earth OR The disintegration, or breakdown of rock material is called Weathering. Three types of weathering: 1)Chemical weathering: breakdown as a result of chemical reactions (CaCO3+CO2+H2O ---> Ca2+ + 2HCO3- ) 2) Mechanical Weathering: No change in chemical composition, just disintegration into smaller pieces 3) Biological Weathering: Can be both chemical and mechanical in nature (For Example: Tree throw). The rate of weathering differs with variation in the chemical composition and structure.
- 19. Chemical Weathering Definition: Transformation/decomposition of one mineral into another Mineral breakdown • carbonate dissolves • primary minerals --> secondary minerals (mostly clays) Water is the main operator: Dissolution Many ionic and organic compounds dissolve in water Silica, K, Na, Mg, Ca, Cl, CO3, SO4 water + carbon dioxide + calcite dissolve into calcium ion and bicarbonate ion H2O + CO2 + CaCO3 --> Ca+2 + 2HCO3 - Acid Reactions Water + carbon dioxide <---> carbonic acid Water + sulfur <--> sulfuric acid H+ effective at breaking down minerals
- 20. Chemical Weathering Oxidation Oxygen dissolved in water promotes oxidation of sulfides, ferrous oxides, native metals Organic Activity Plant material makes H+ ions available Hydration Attachment of water molecules to crystalline structure of a rock, causing expansion and weakness Hydrolysis combination of hydrogen and oxygen in water with rock to form new substances
- 21. Factors that Influence Chemical Weathering The factors that influence Chemical weathering are, The climate of the place (Temperature and moisture characteristics). The vegetation (Most effective in areas of warm, moist climates – decaying vegetation creates acids that enhance weathering) The physical nature of the rock. In case of Chemical weathering water plays a major role as is evident from the description of methods, therefore in the absence of water, chemical weathering is nearly impossible.
- 22. TYPES OF WEATHERING REACTIONS
- 23. Geochemical analysis Geochemistry is the study of the composition of geological materials and the behavior of individual elements during geological processes. Geochemical analysis is now a vital tool in most fields of geological and environmental research. It is used, for example, in studies of water, soil, and air quality, of formation of rocks and minerals, of fossilization mechanisms, of metal accumulation in organisms from contaminated water and soil. Some of the most commonly available geochemical techniques are described below. Electron probe microanalysis (EPMA) X-ray fluorescence spectrometry (XRF)
- 24. Electron probe microanalysis (EPMA) EPMA is a non-destructive technique for the analysis of polished and carbon-coated specimens of minerals, glasses, and synthetic materials. It can also be used to give an indication of the elements present in organic or unpolished or uncoated materials. Analyses can be made at individual points (usually 1 to 40 m (micrometres) inμ diameter) or over small areas (usually less than 1 mm2 ) of the sample surface to produce geochemical maps. Specimens are introduced into the sample vacuum chamber and viewed under high magnification to select areas of interest. A beam of electrons is fired at the surface of the sample, producing X-rays with energies and wavelengths specific to the elements present.
- 25. Electron probe microanalysis (EPMA) The instrument is calibrated by analyzing standard samples with known compositions. Among the most useful applications of EPMA is in the study of subtle chemical zoning in minerals by element mapping. Zoning of this kind, which is often invisible using light microscopy, can yield information on crystallization conditions and changes in the chemistry of a magma or hydrothermal fluid.
- 26. X-ray fluorescence spectrometry (XRF) XRF is a routine technique for the determination of major elements and many trace elements in rocks and minerals, at concentrations from 1 or 2 ppm (parts per million) to 100 per cent. Samples are usually prepared as glass discs for major element analyses, by fusing the sample powder with a known proportion of a commercially available flux, or as pressed powder pellets for trace-element analyses, made by mixing the sample powder with a binding agent, then pressing the mixture into a compact disc with a smooth upper surface. The sample surface is irradiated with primary X-rays, producing secondary X-rays with energies and wavelengths characteristic of the elements present.
- 27. X-ray fluorescence spectrometry (XRF) The concentration of the elements is determined by comparing the intensity of the various energy or wavelength peaks with those produced by standard samples of known composition. One of the most common uses of XRF is in the geochemical analysis of suites of igneous, metamorphic, and sedimentary rocks in studies of crustal and mantle evolution.
- 28. The interpretation of geochemical data Data for major elements are generally reported in the form of oxides as weight per cent (e.g. silica, SiO2 wt. percent) and trace elements in parts per million (ppm) or micrograms per gram ( gμ g 1− ). An important consideration in the interpretation of data is analytical uncertainty, particularly when evaluating the existence or significance of small variations in composition. Some of the most common sources of uncertainty are sample contamination, incomplete sample digestion (some minerals are extremely resistant to acids), interferences between elements (for example in EPMA, where X-ray energies for two elements may overlap), and poor instrument calibration.
- 29. The interpretation of geochemical data The extent of these problems is best limited at the time of analysis by taking necessary precautions and through discussion with experienced laboratory staff. They are, however, impossible to eliminate completely and an indication of analytical uncertainty should therefore be reported with the data, ideally as ‘error bars’ on geochemical diagrams. Uncertainties can be estimated from the regular analysis of reference materials of known composition and from the analysis of two or more samples. To help in interpretation, data are often imported into graphing and statistical computer programmes. A wide variety of diagrams can be produced to compare the data with those from previous studies and to demonstrate trends or new interpretations.
