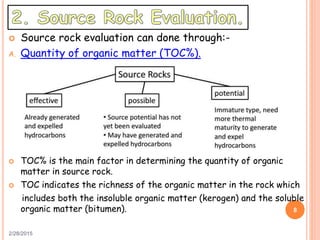
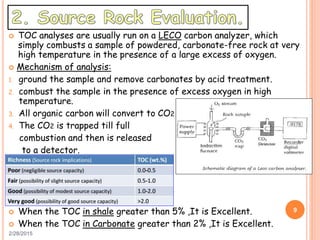
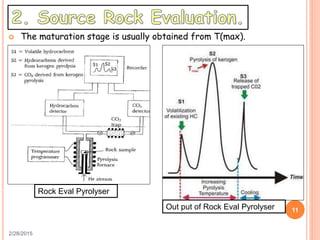
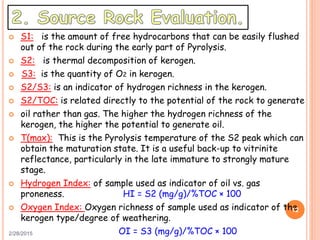
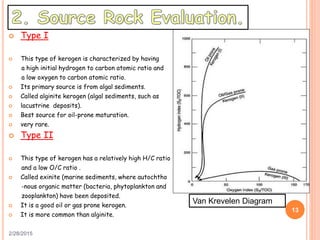
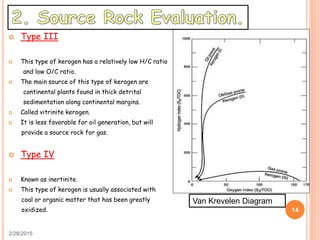
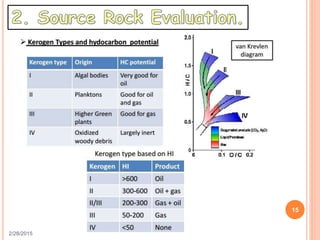
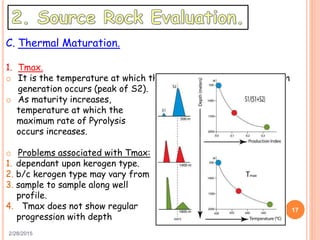
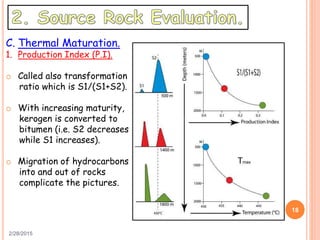
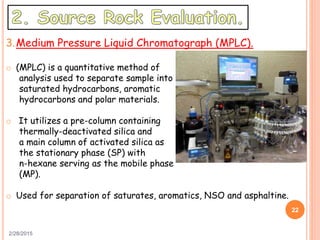
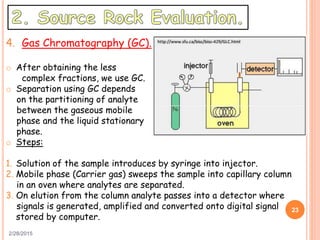
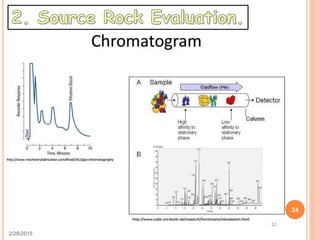
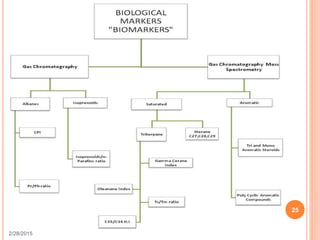
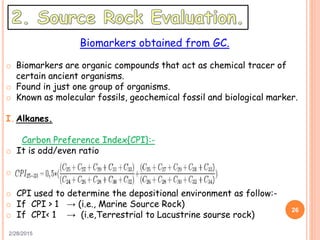
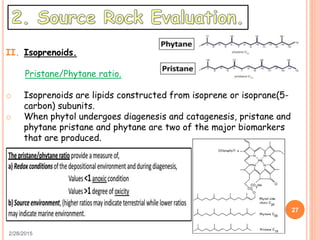
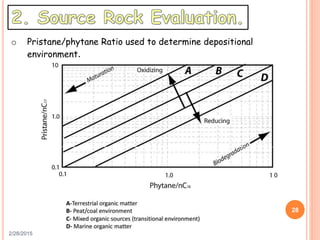
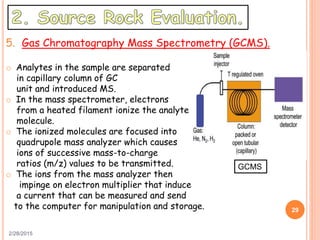
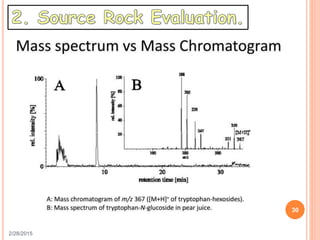
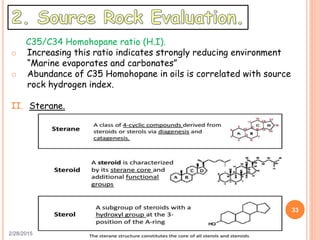
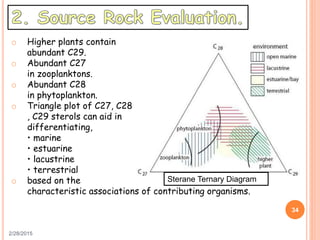
This document discusses the use of organic geochemistry in oil exploration. It begins with an introduction to organic geochemistry and outlines source rock evaluation including quantity and quality of organic matter, and thermal maturation. Quantity is evaluated using total organic carbon content. Quality is evaluated using Rock-Eval pyrolysis and van Krevelen diagrams to determine kerogen type. Maturation is evaluated using Tmax, vitrinite reflectance and production index. Biomarkers obtained through extraction, chromatography and mass spectrometry are used to determine depositional environment.