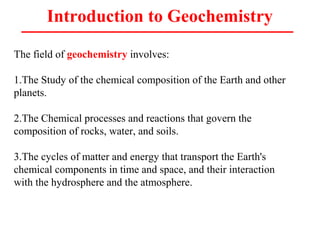
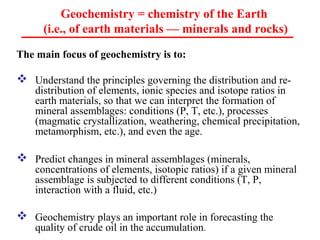
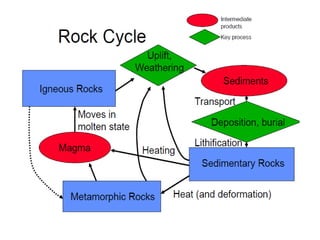
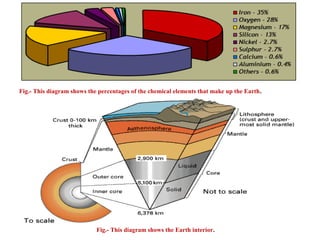
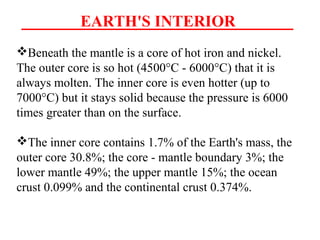
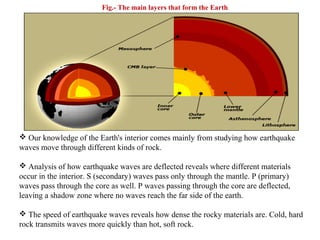
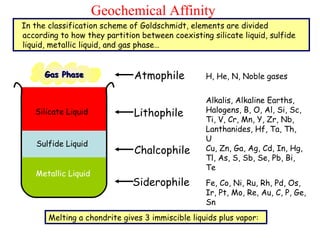
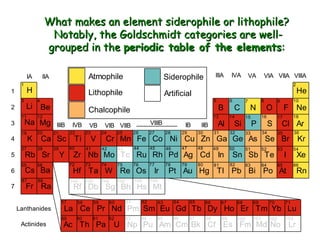
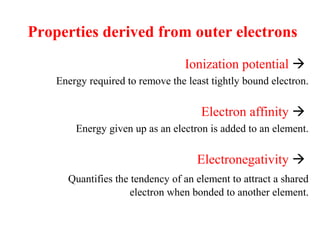
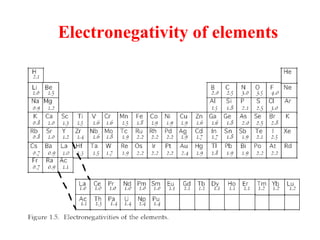
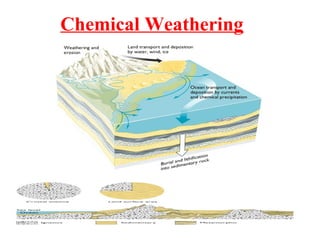
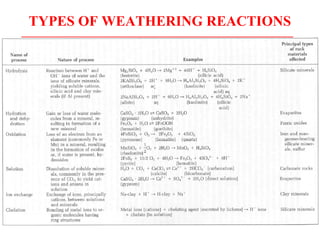
Geochemistry involves studying the chemical composition of Earth and other planets, as well as the chemical processes that govern rocks, water, and soils. It examines how chemical elements are distributed and move through different parts of Earth over time. Key techniques for geochemical analysis include electron probe microanalysis and X-ray fluorescence spectrometry. Proper interpretation of geochemical data requires considering analytical uncertainty and discussing limitations with laboratory experts.