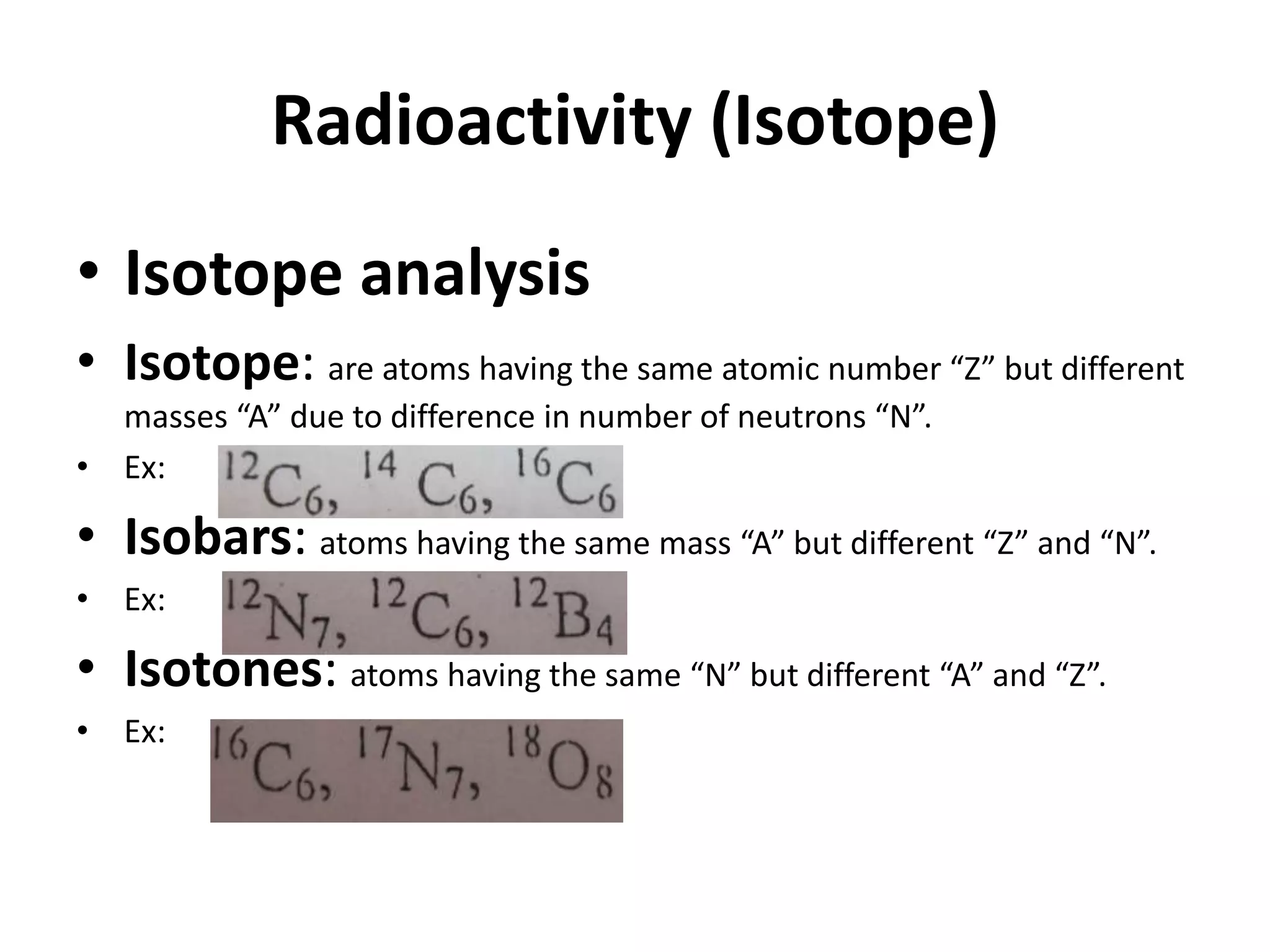
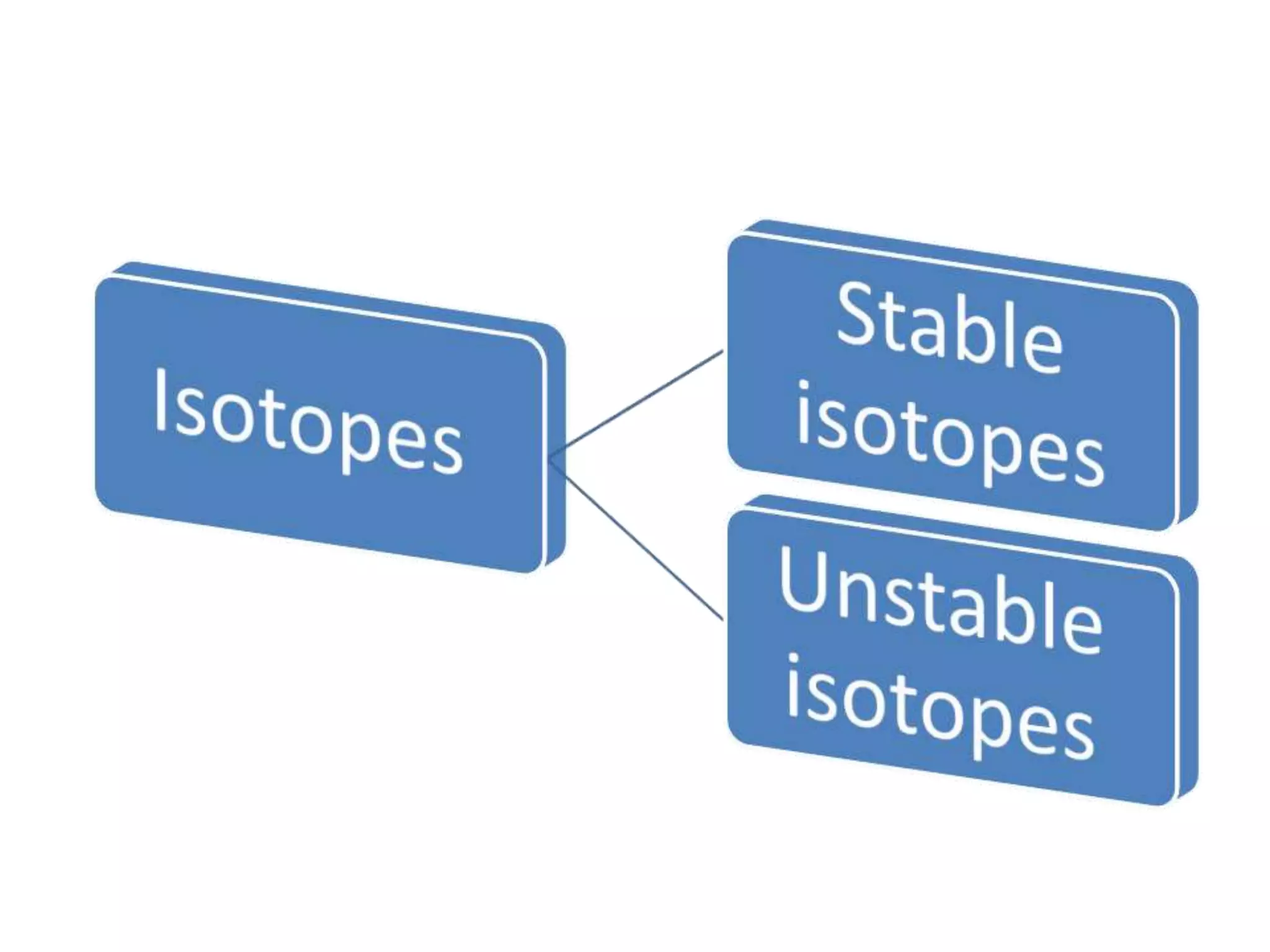
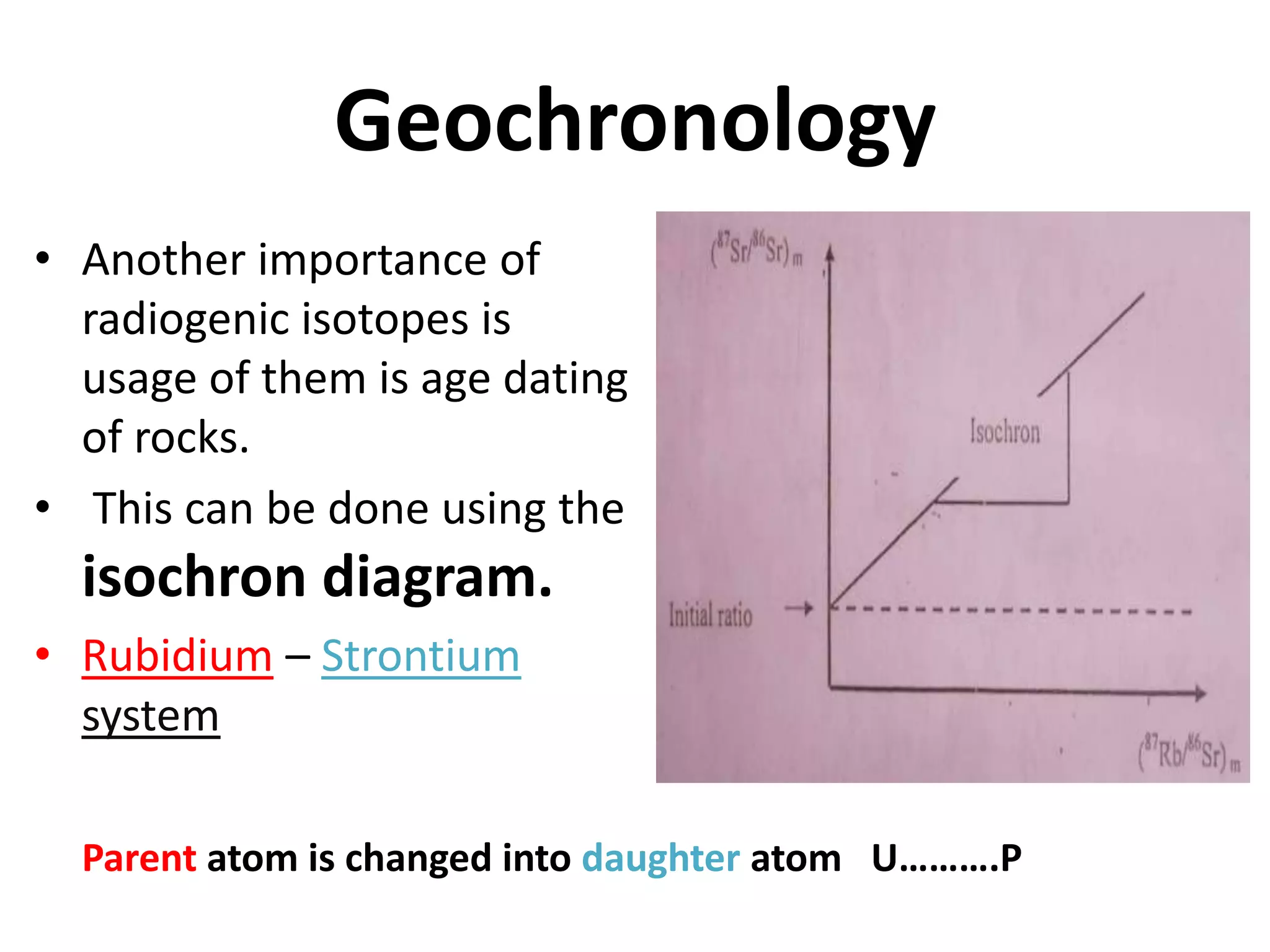
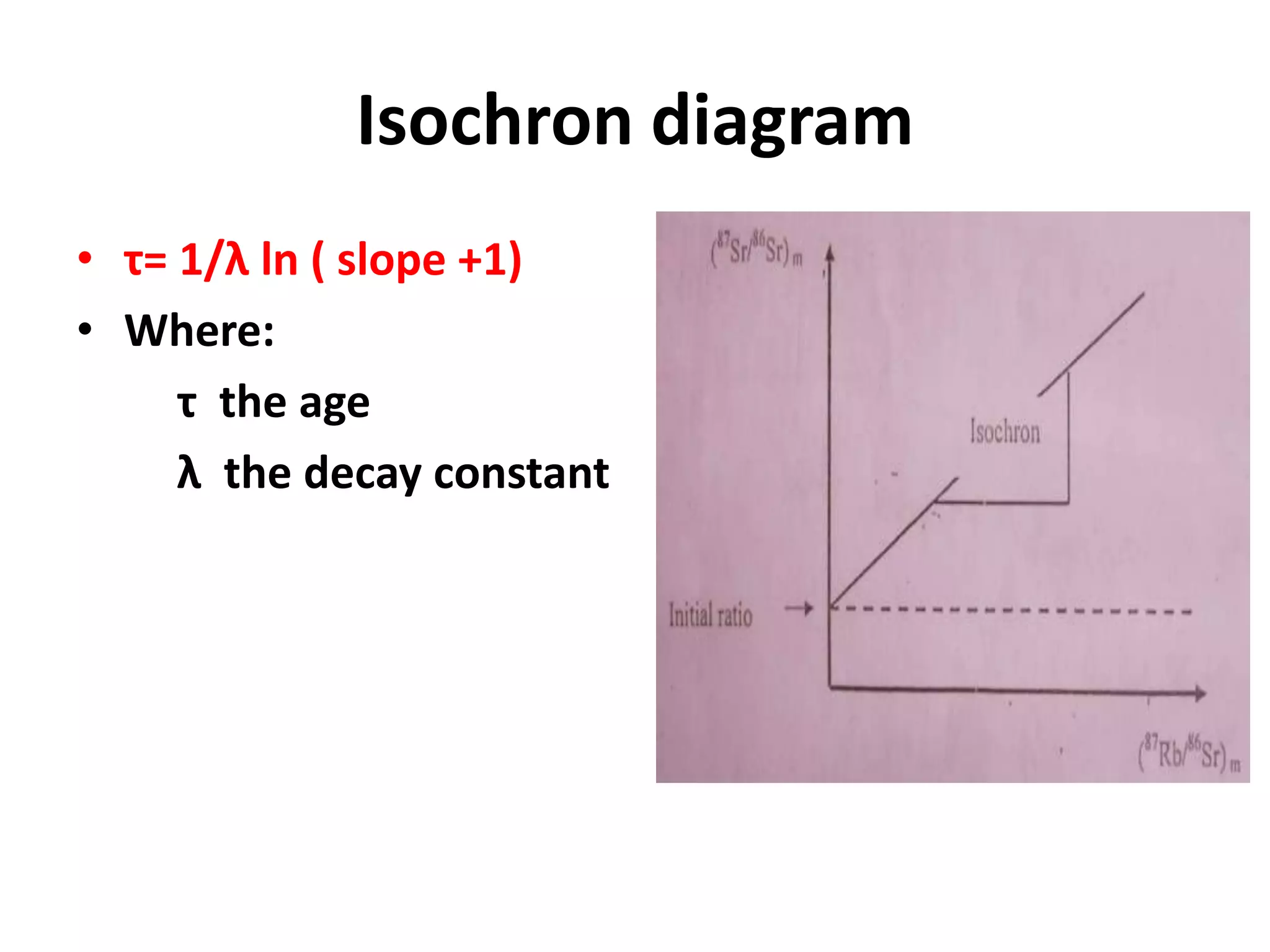
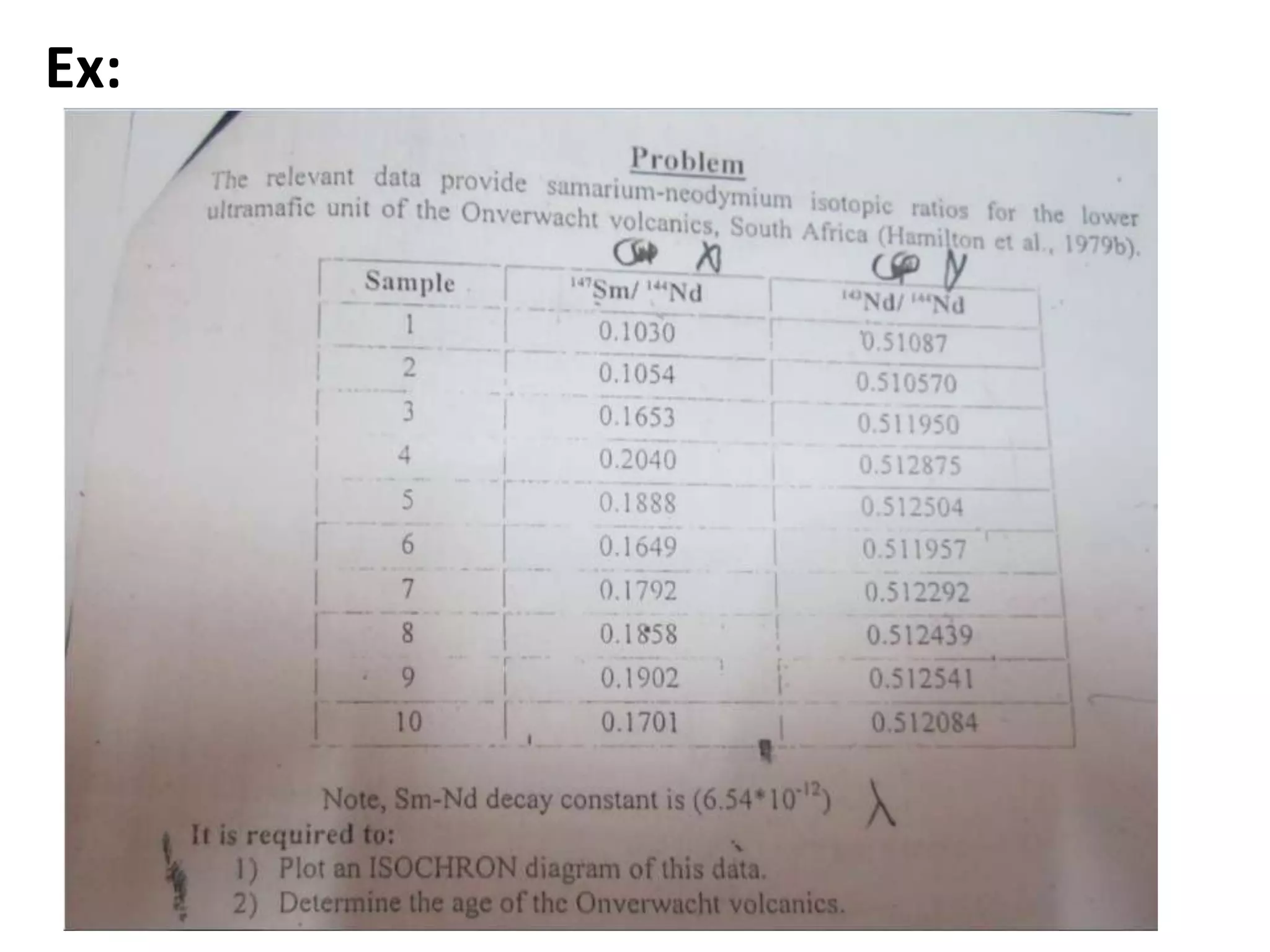
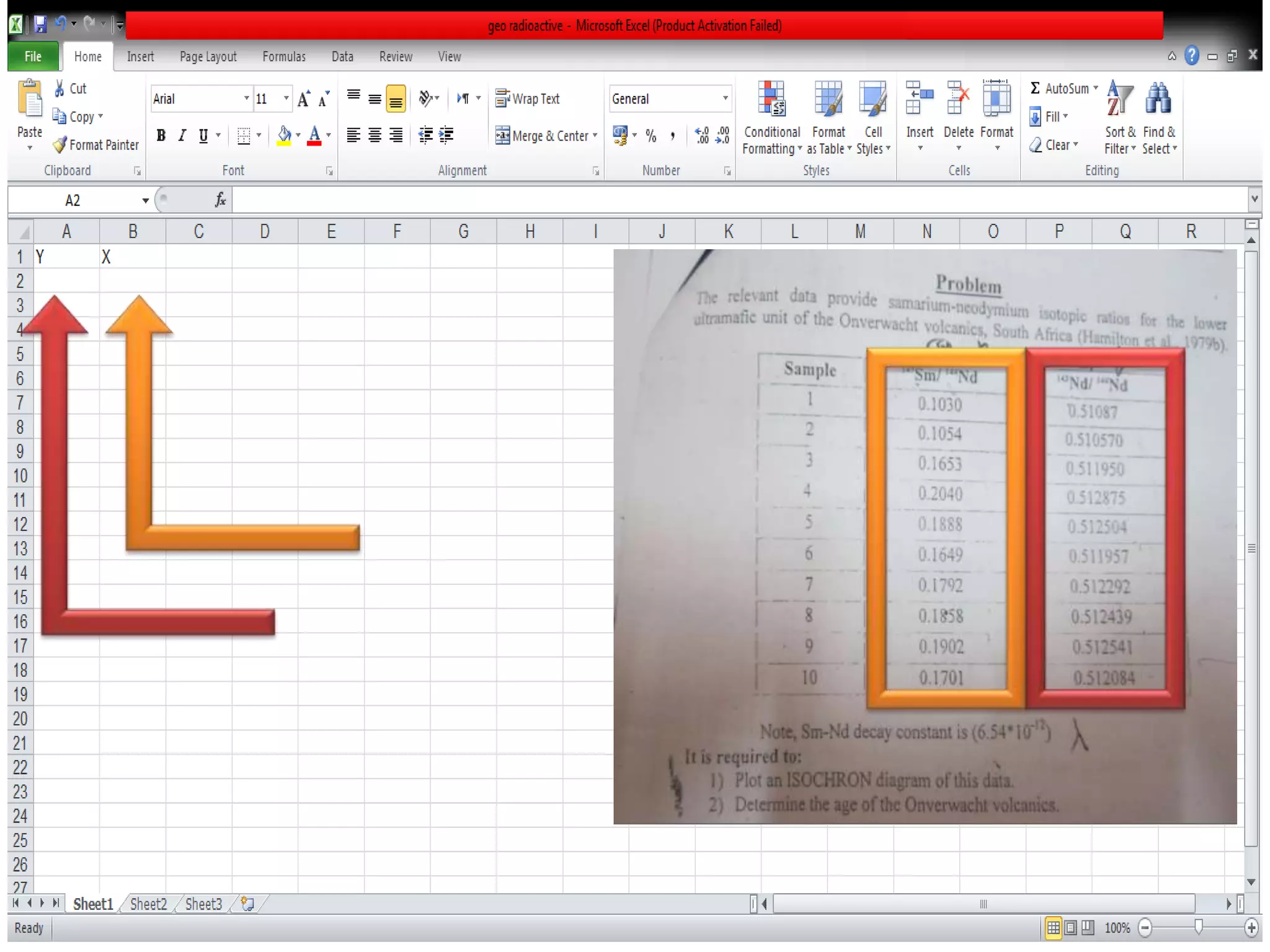
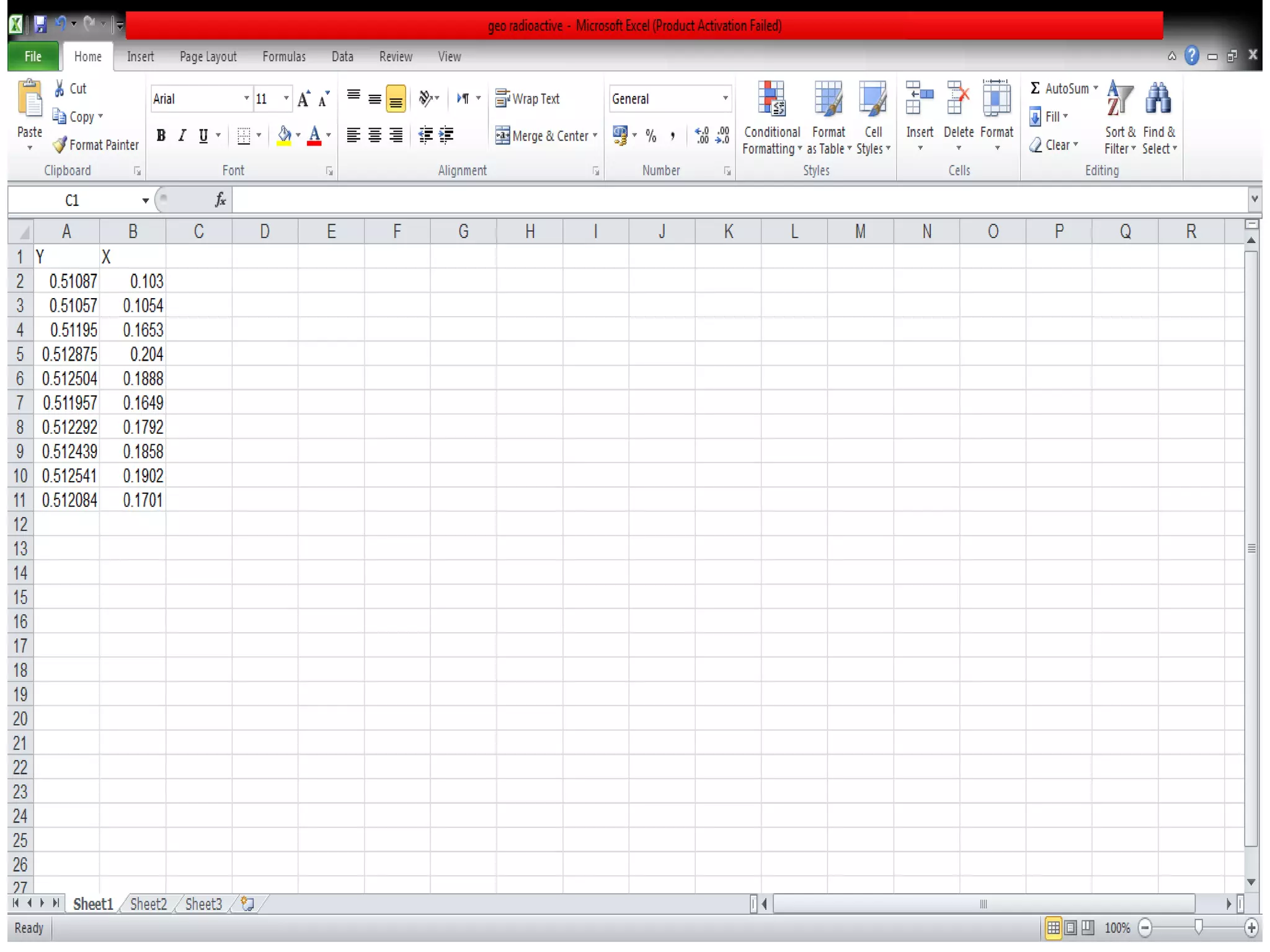
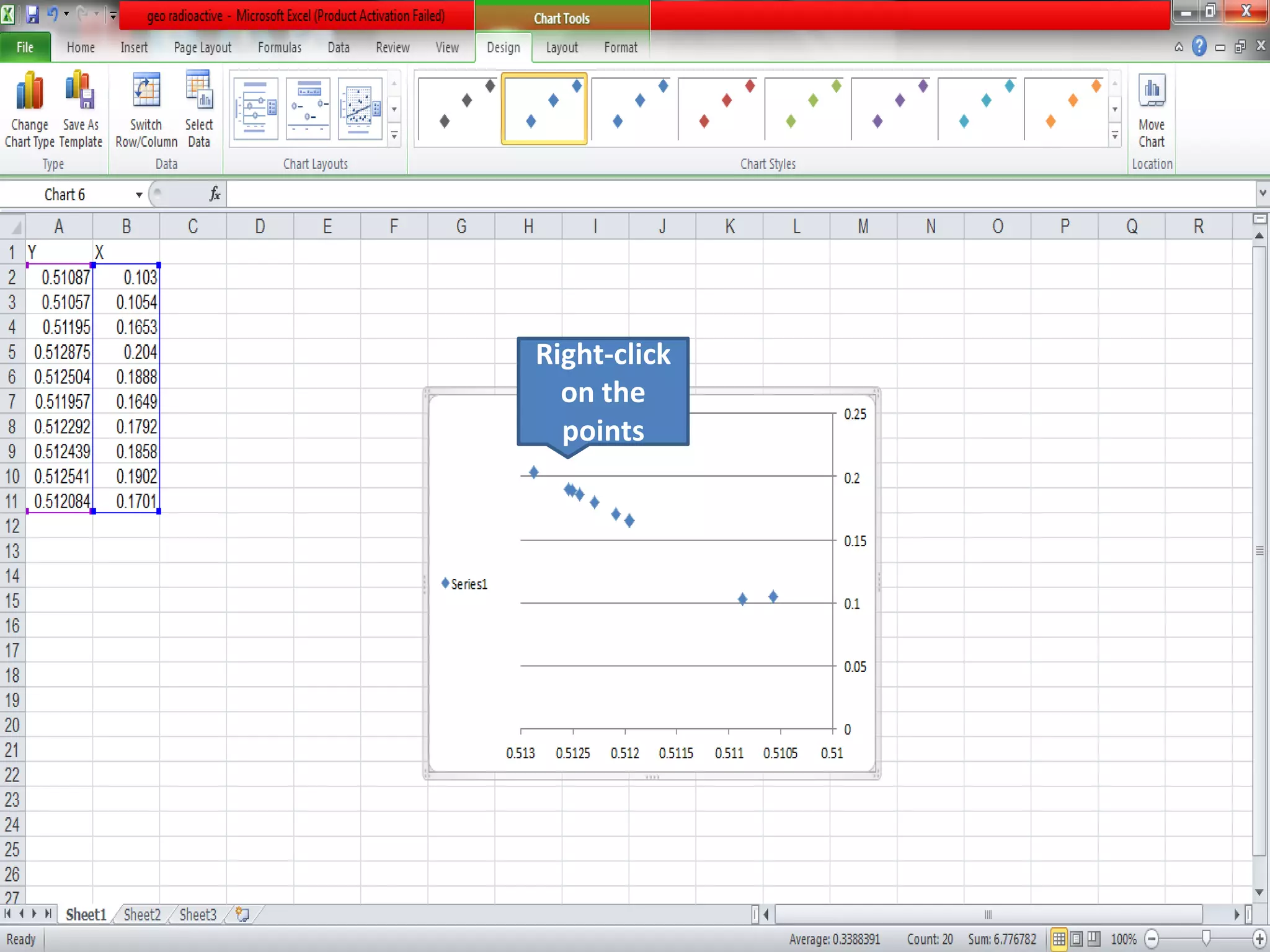
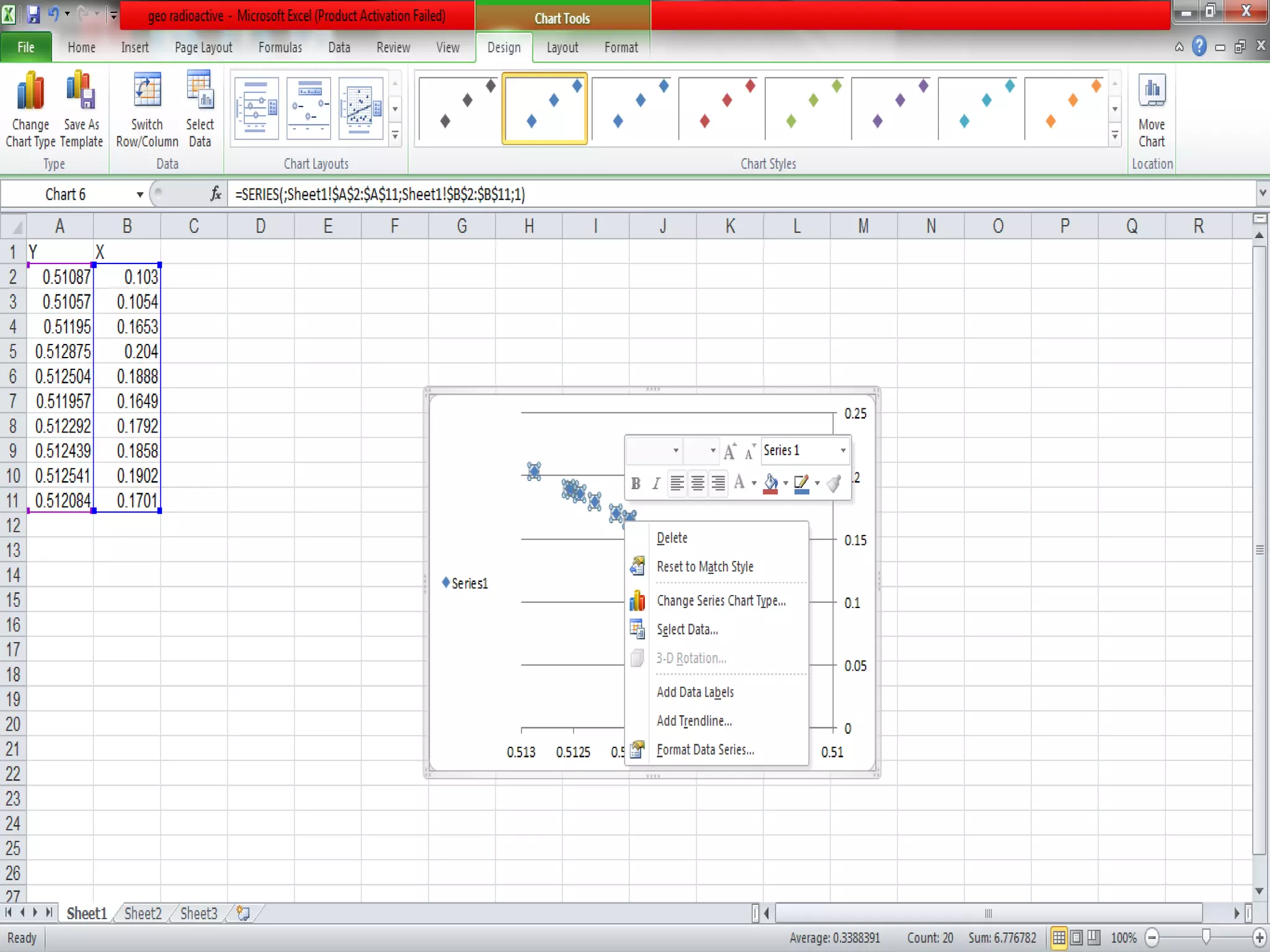
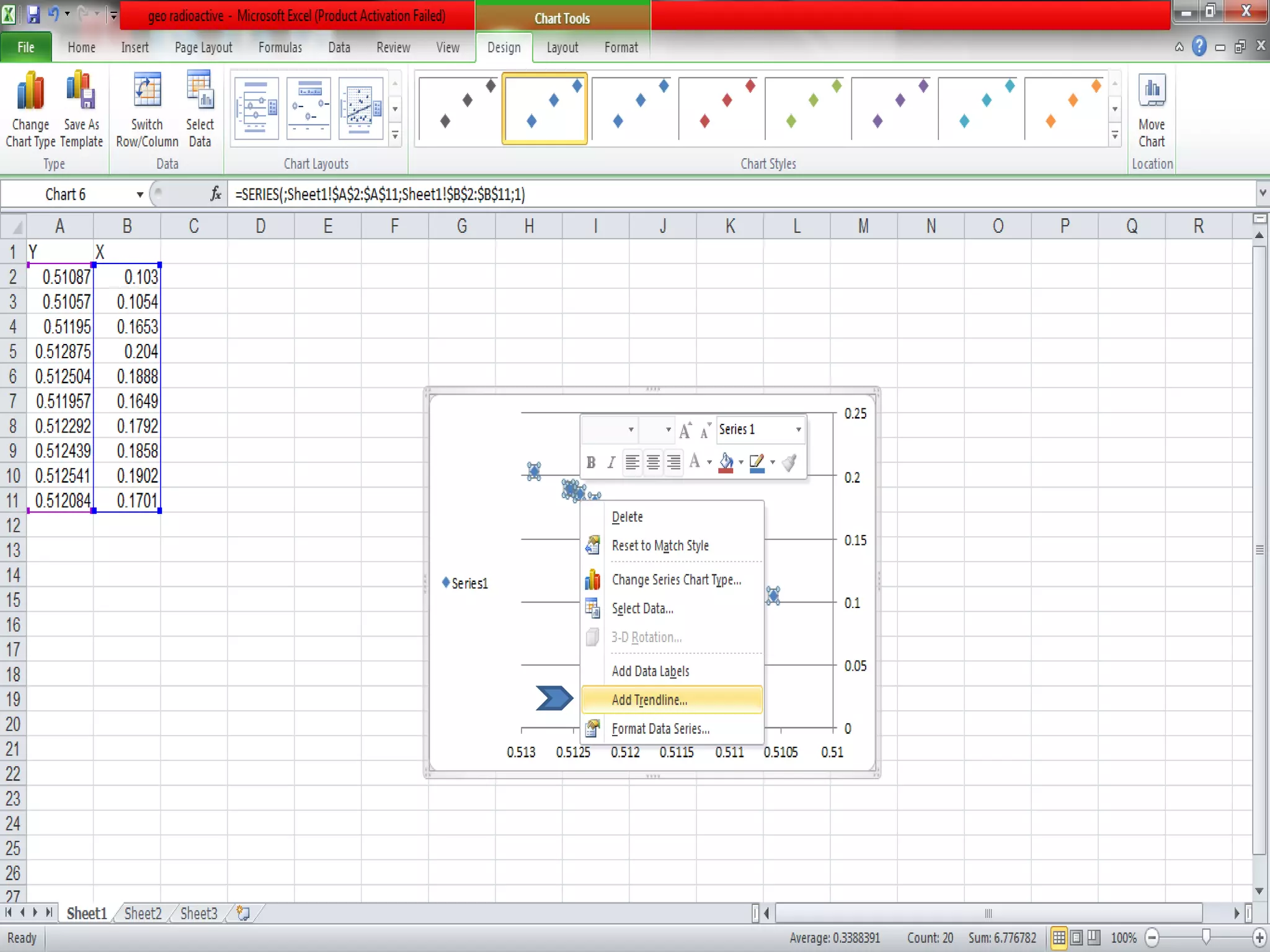
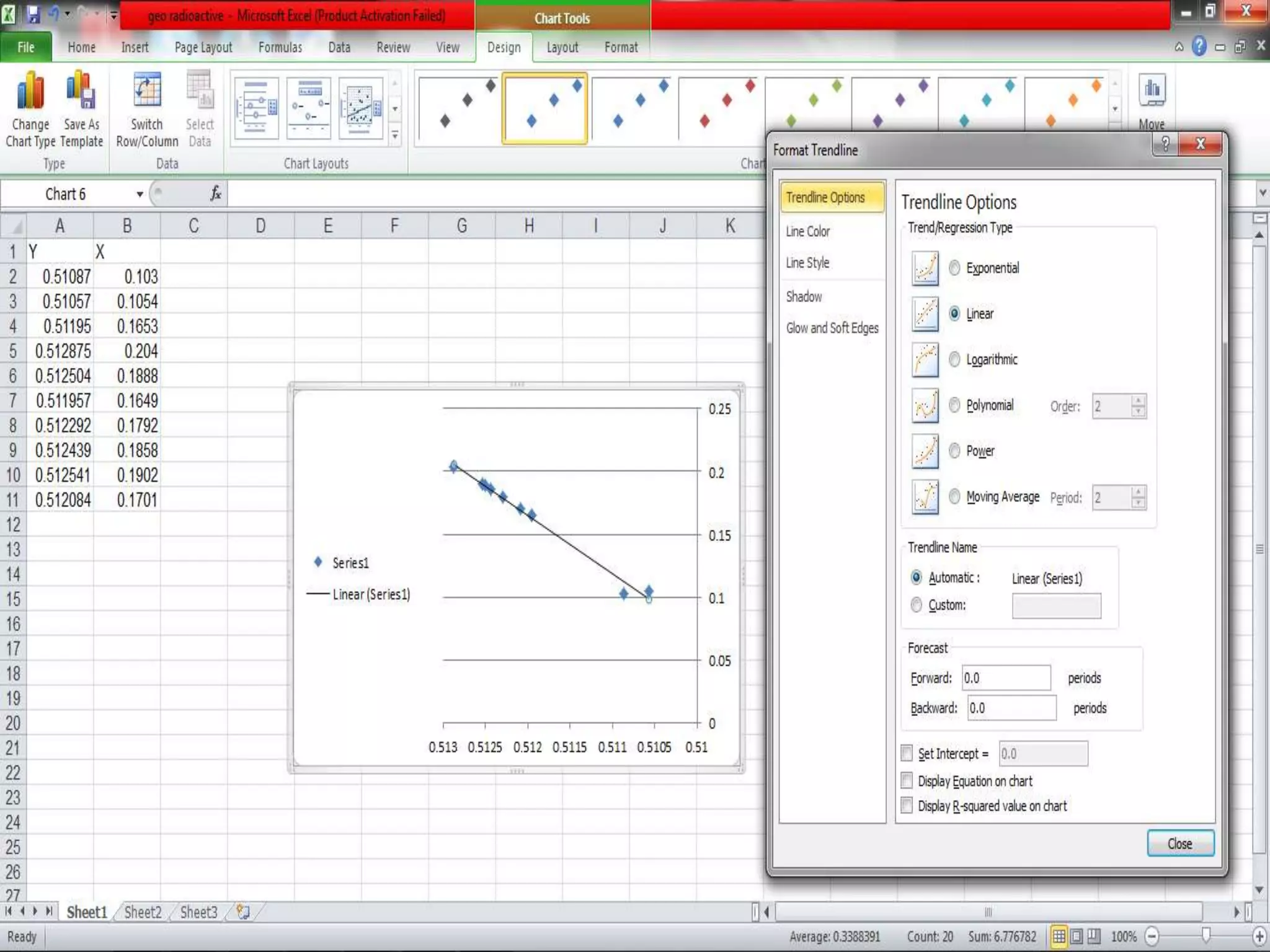
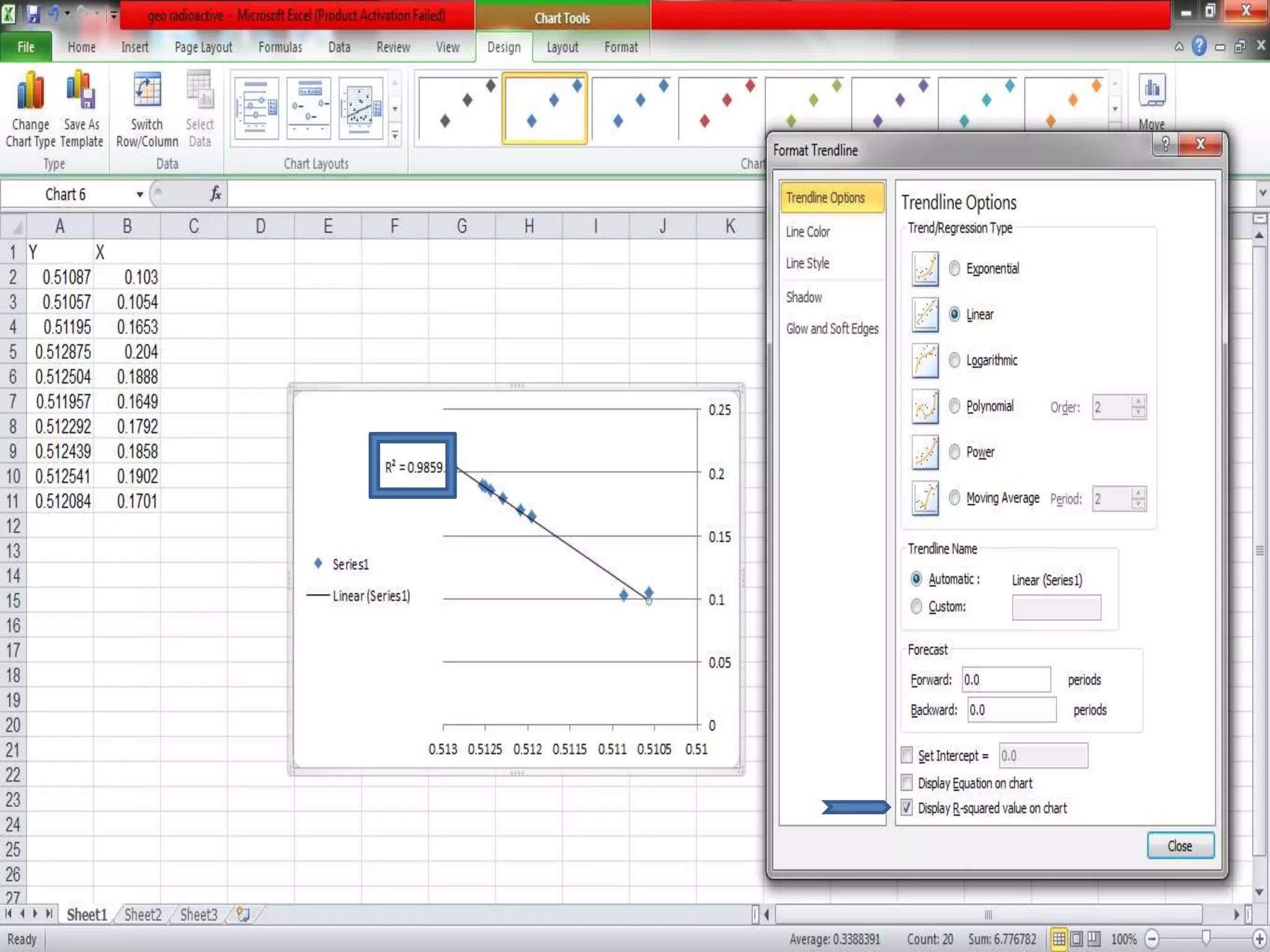
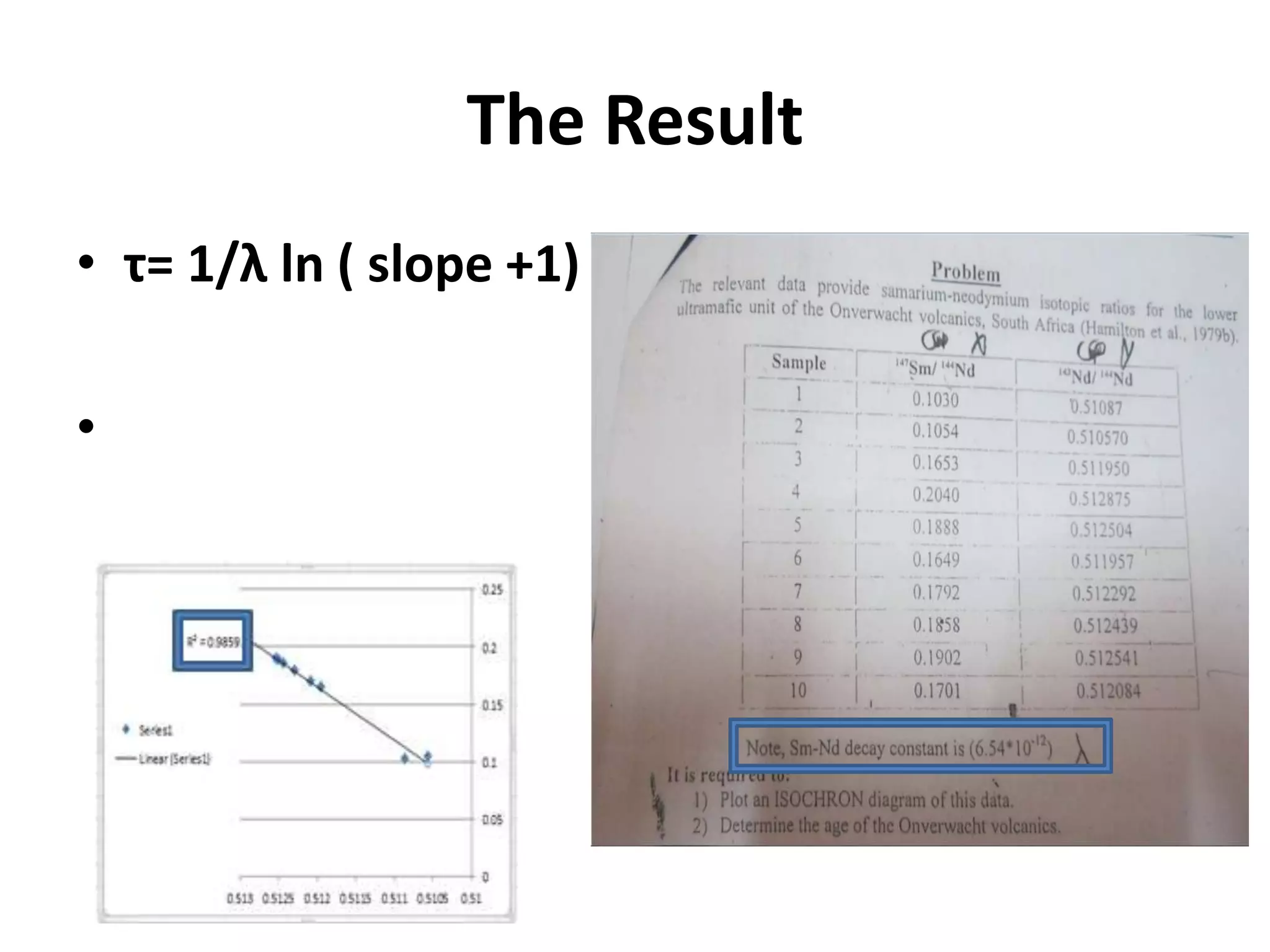
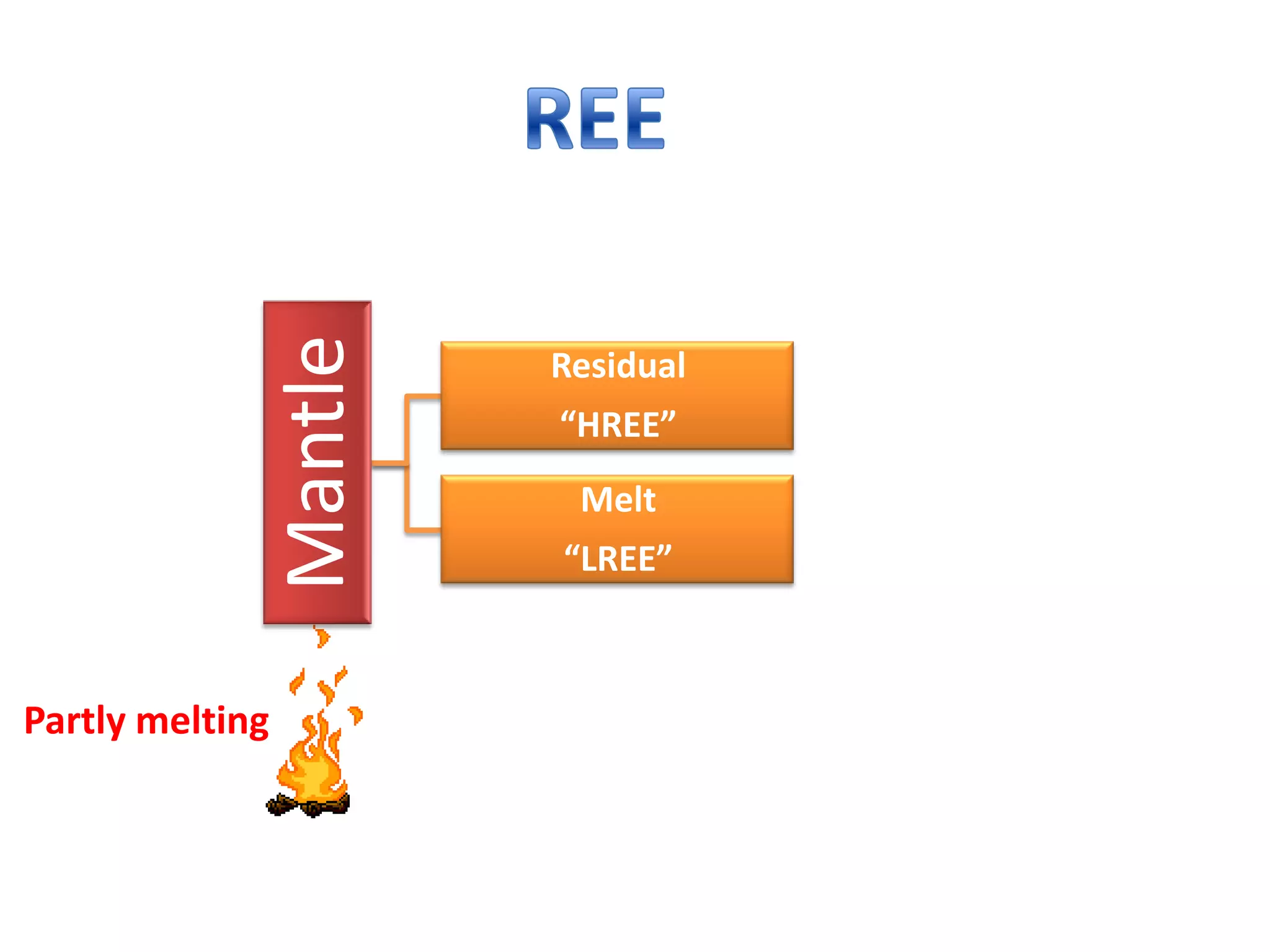
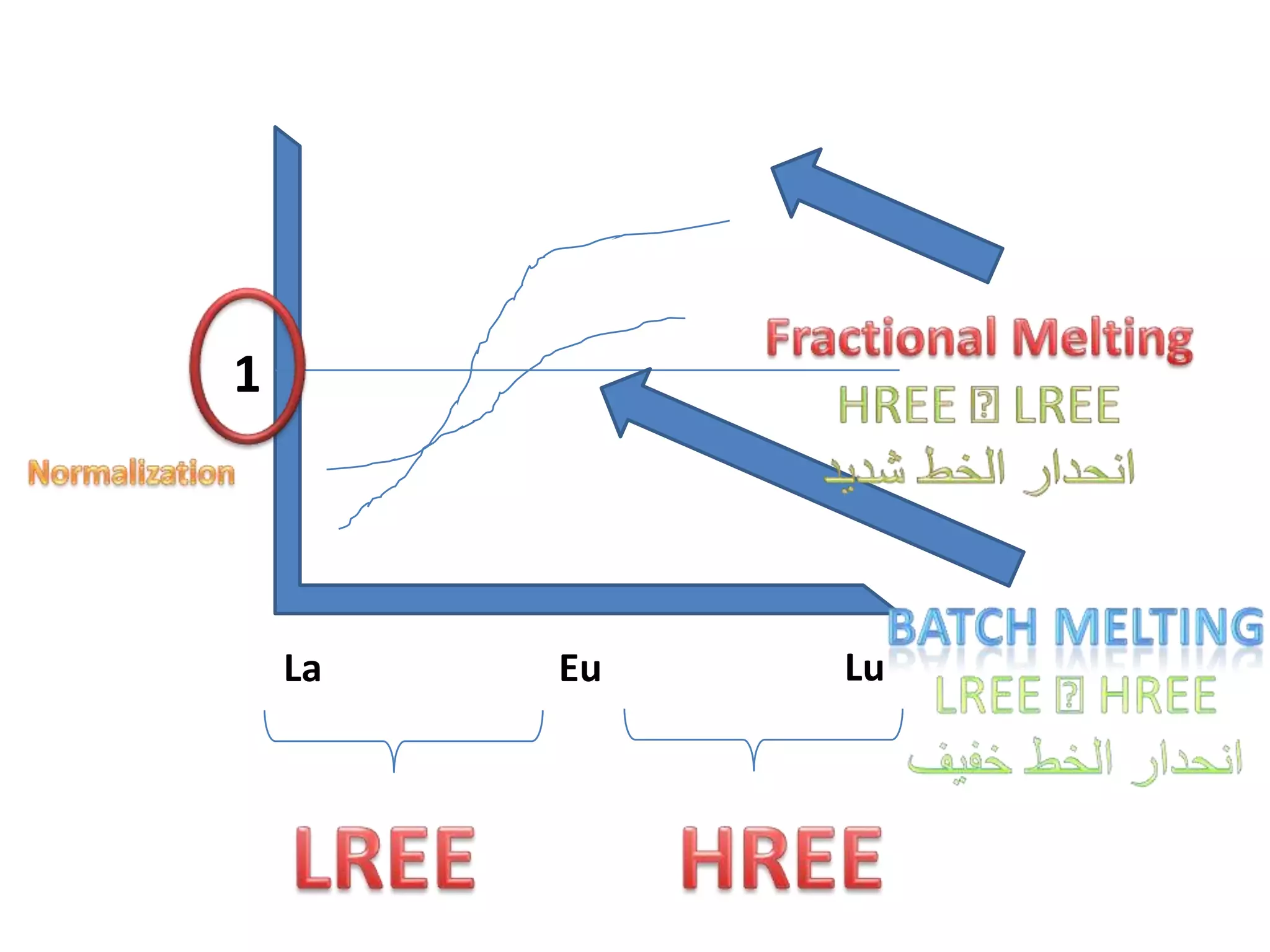
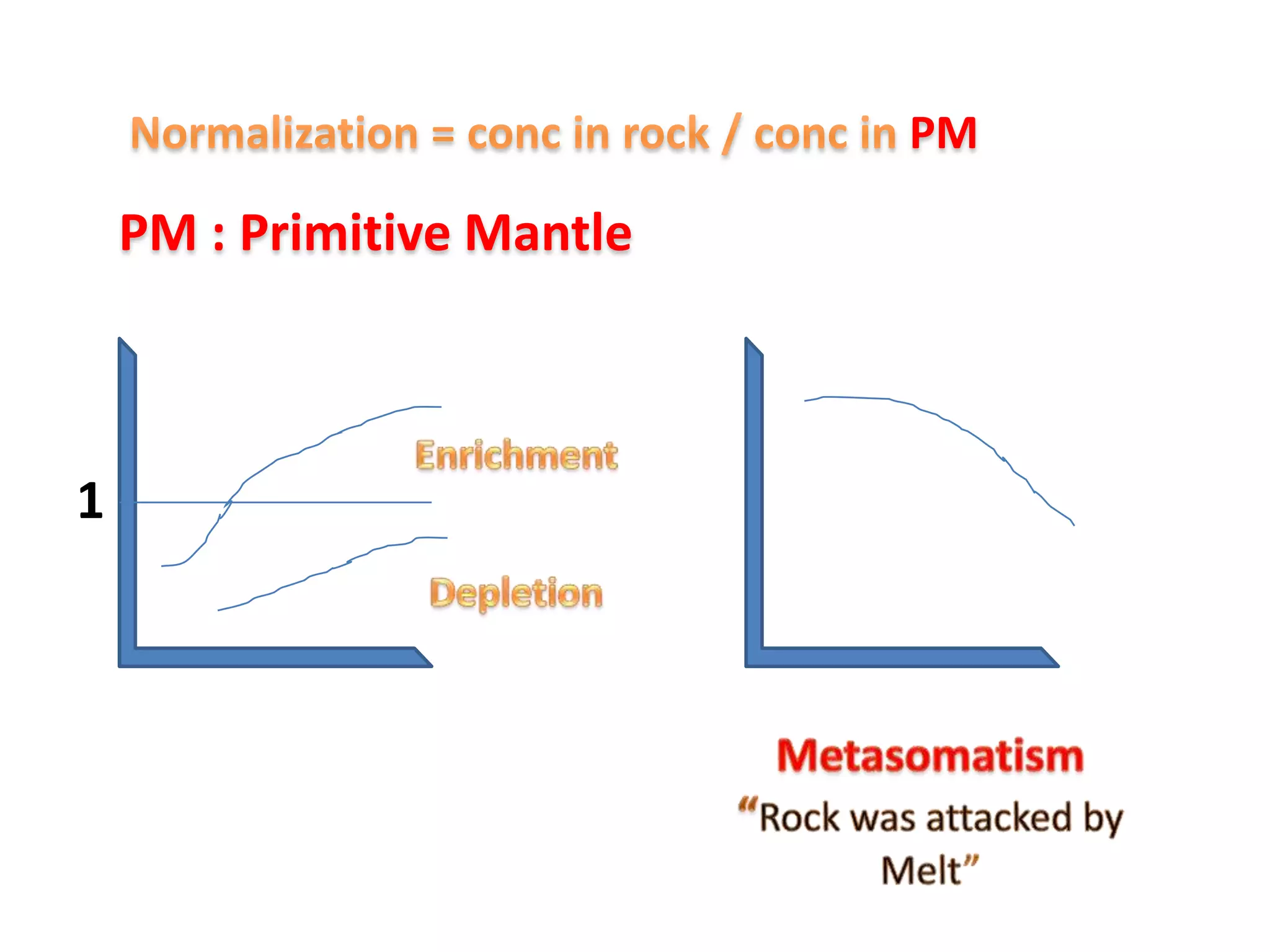
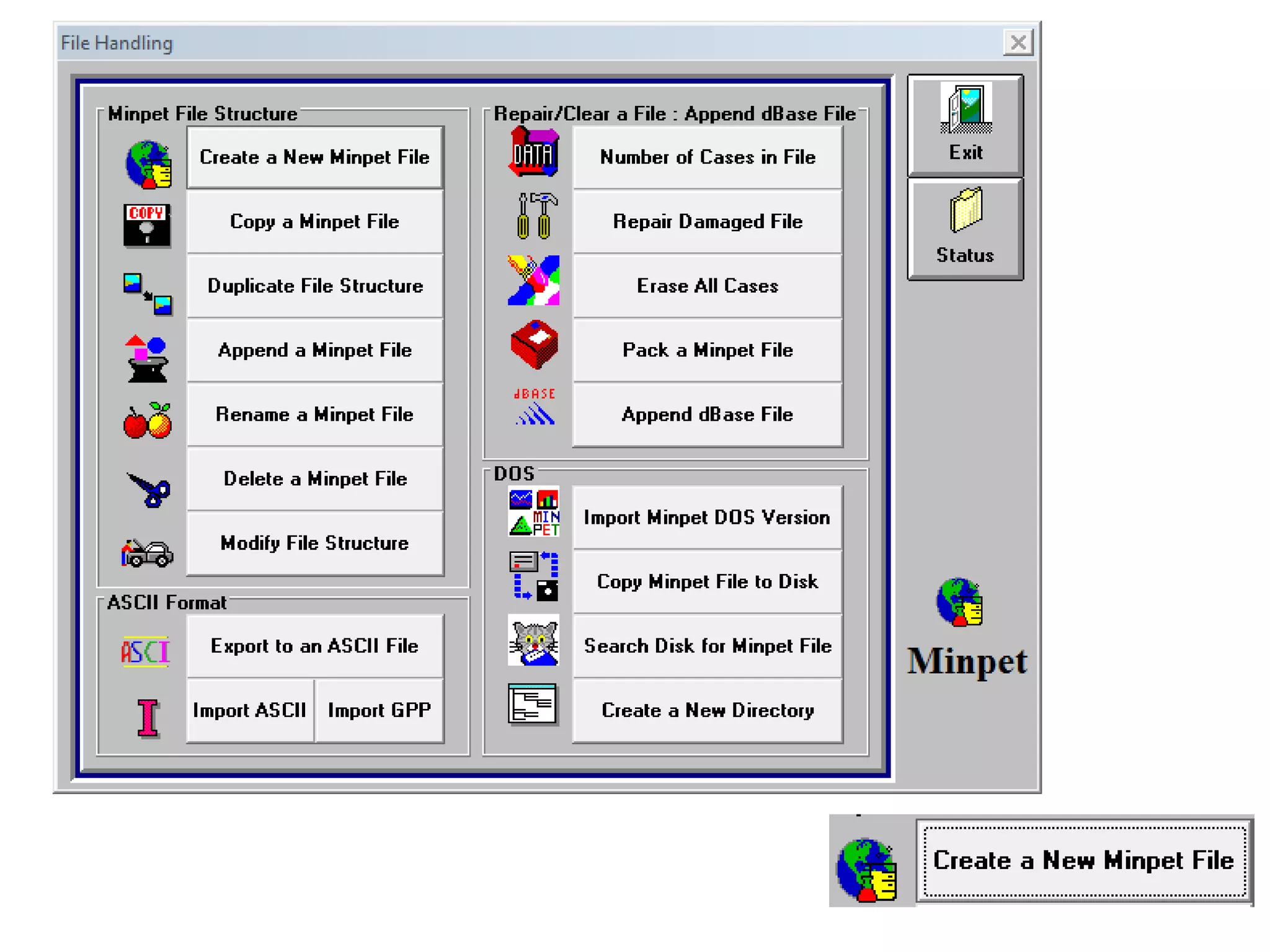
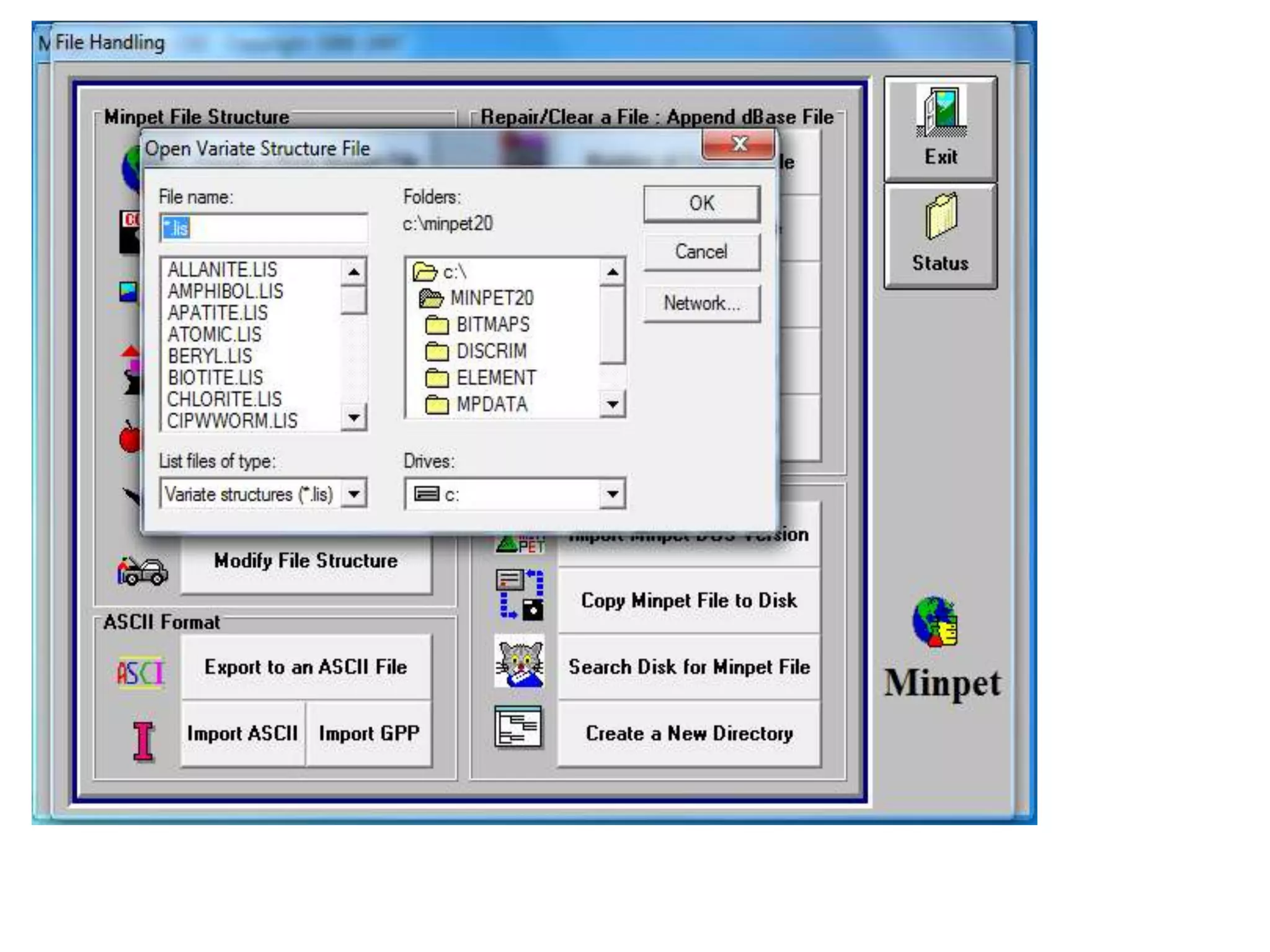
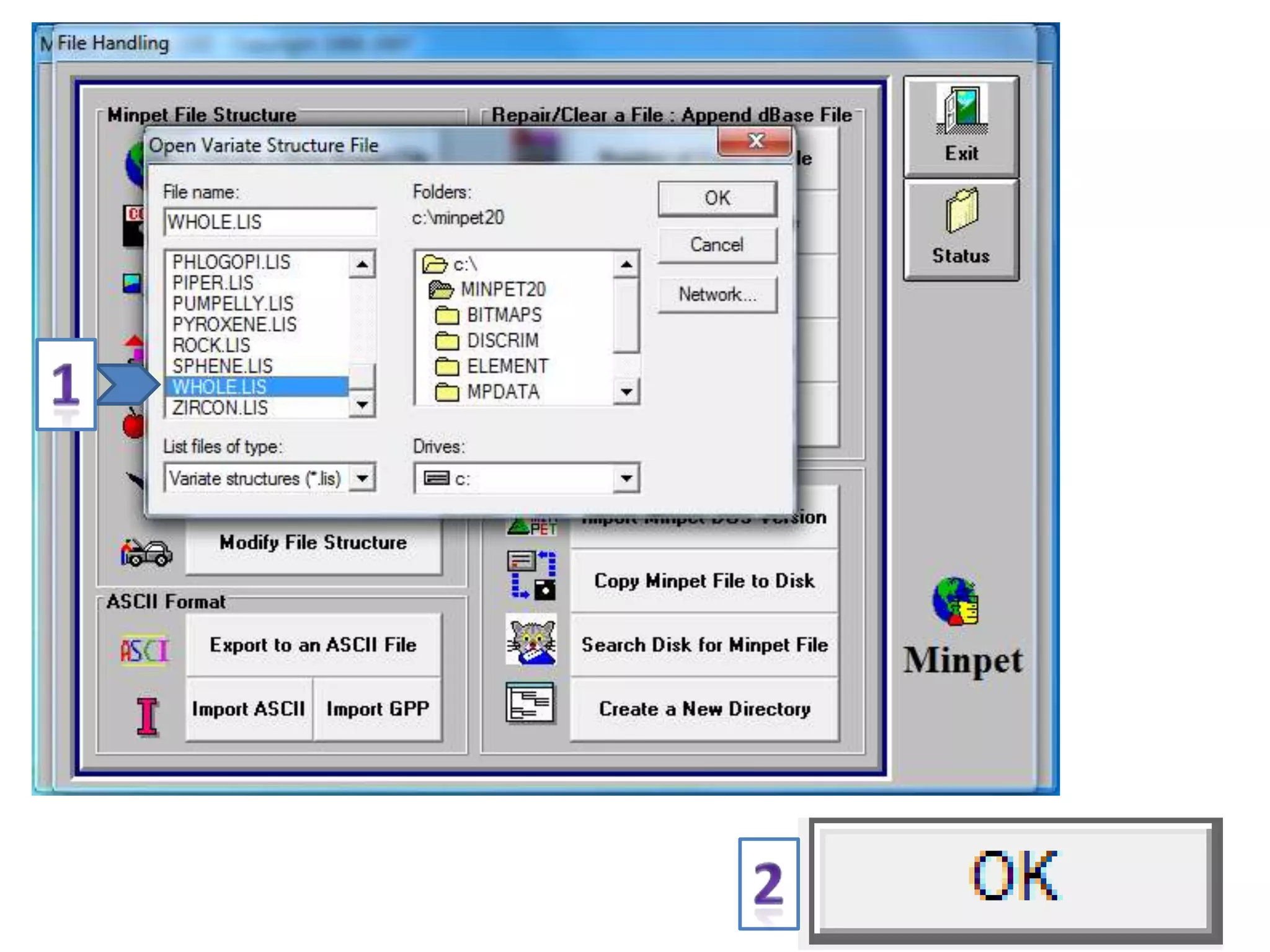
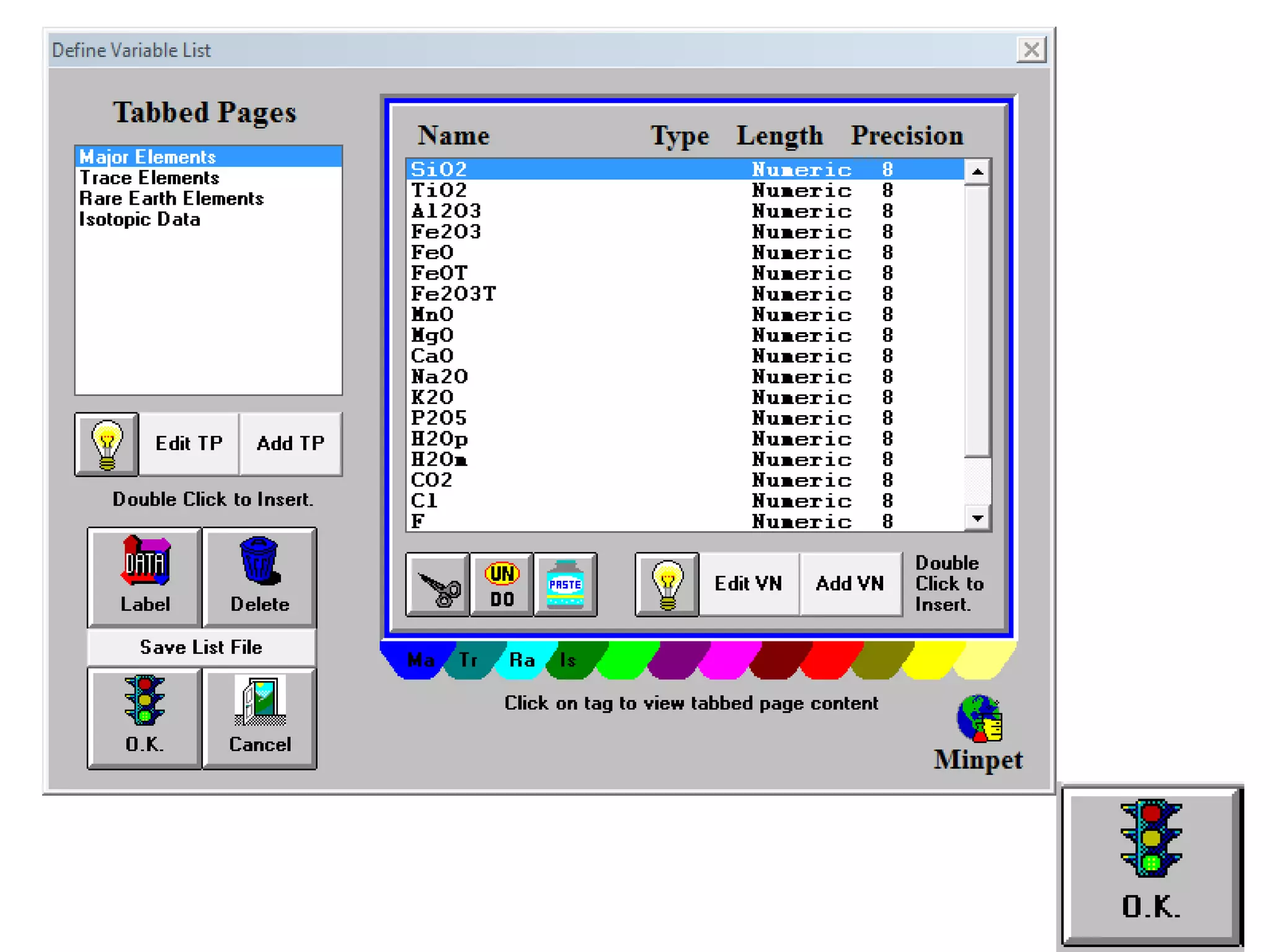
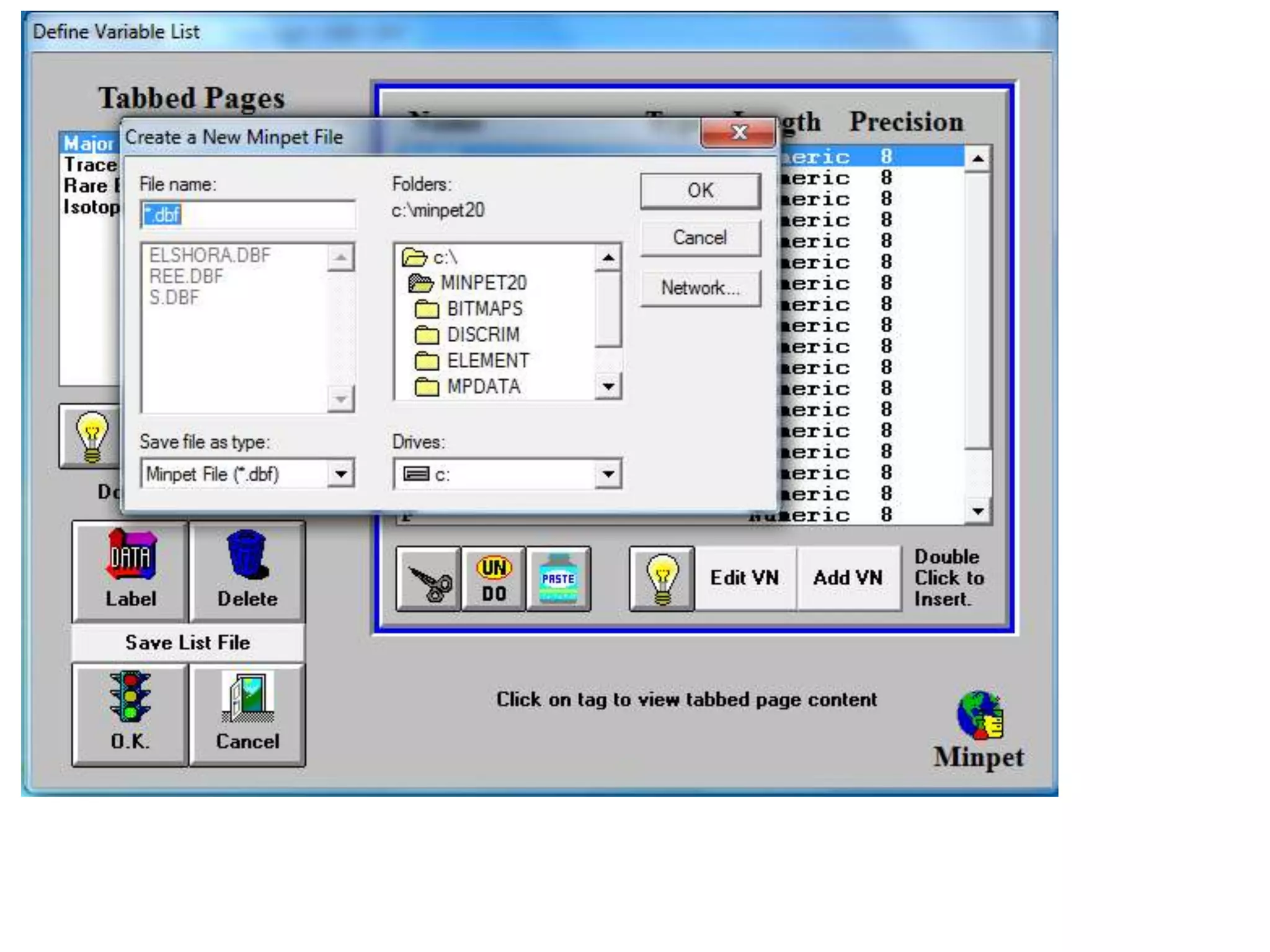
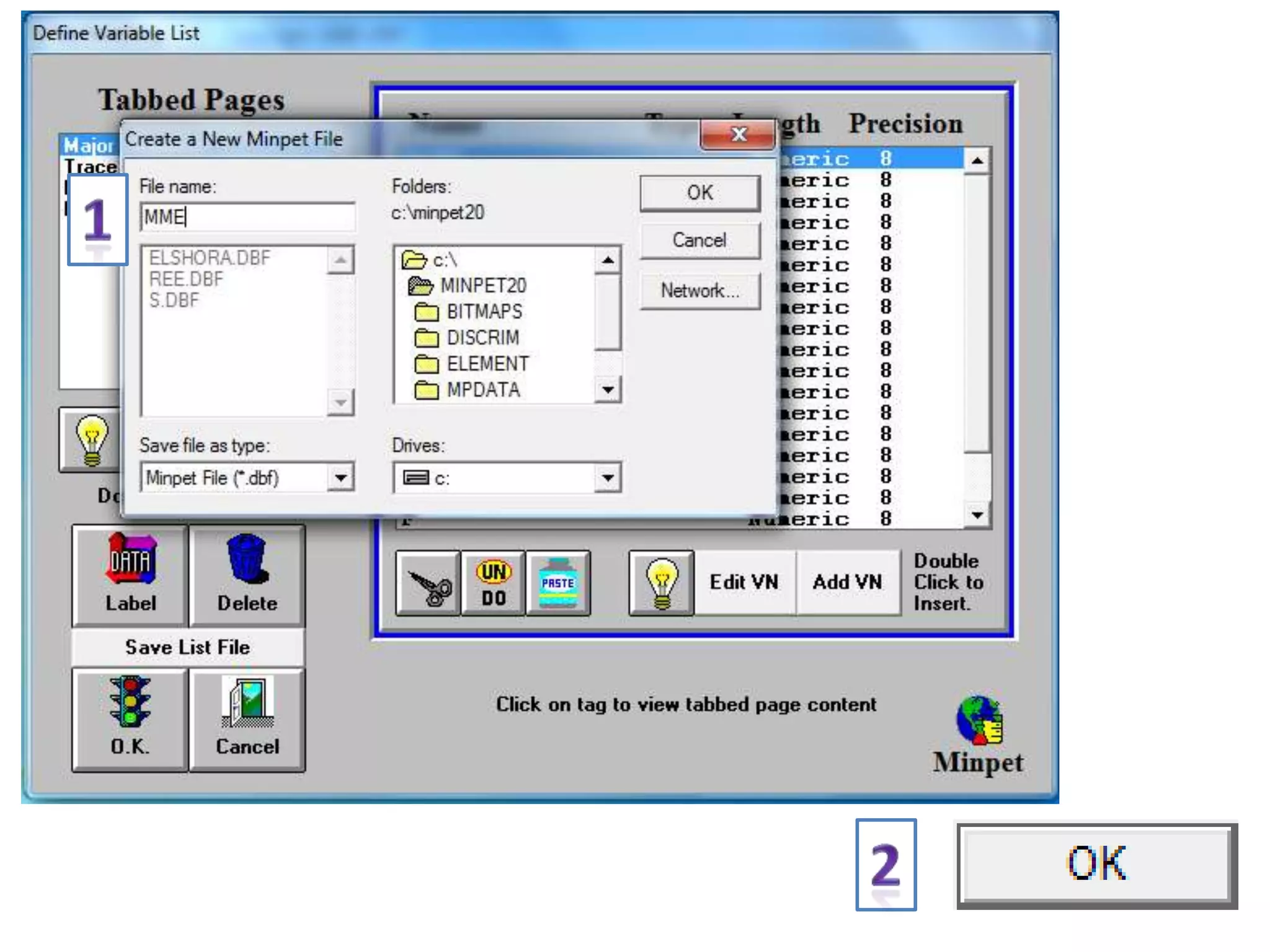
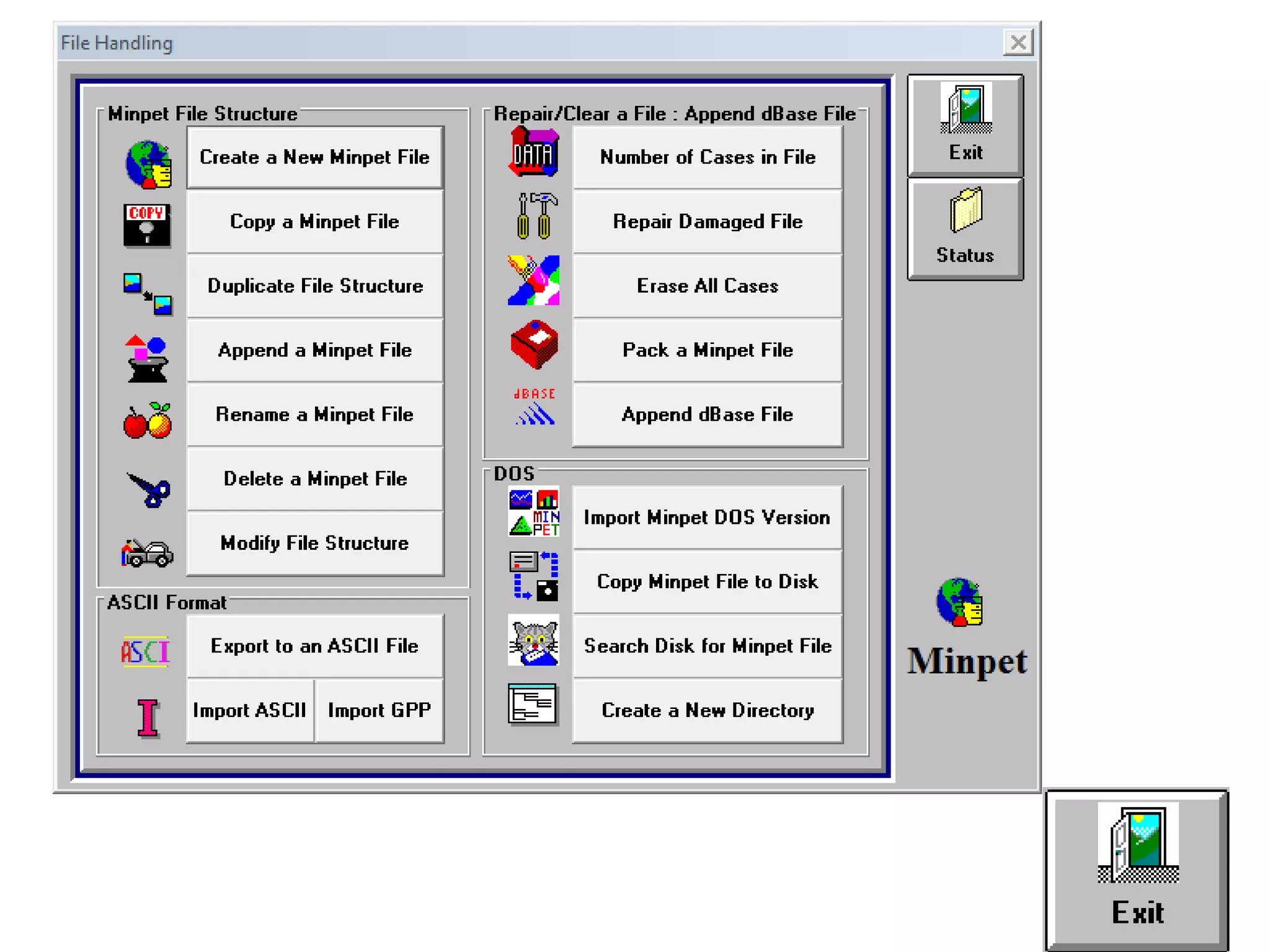
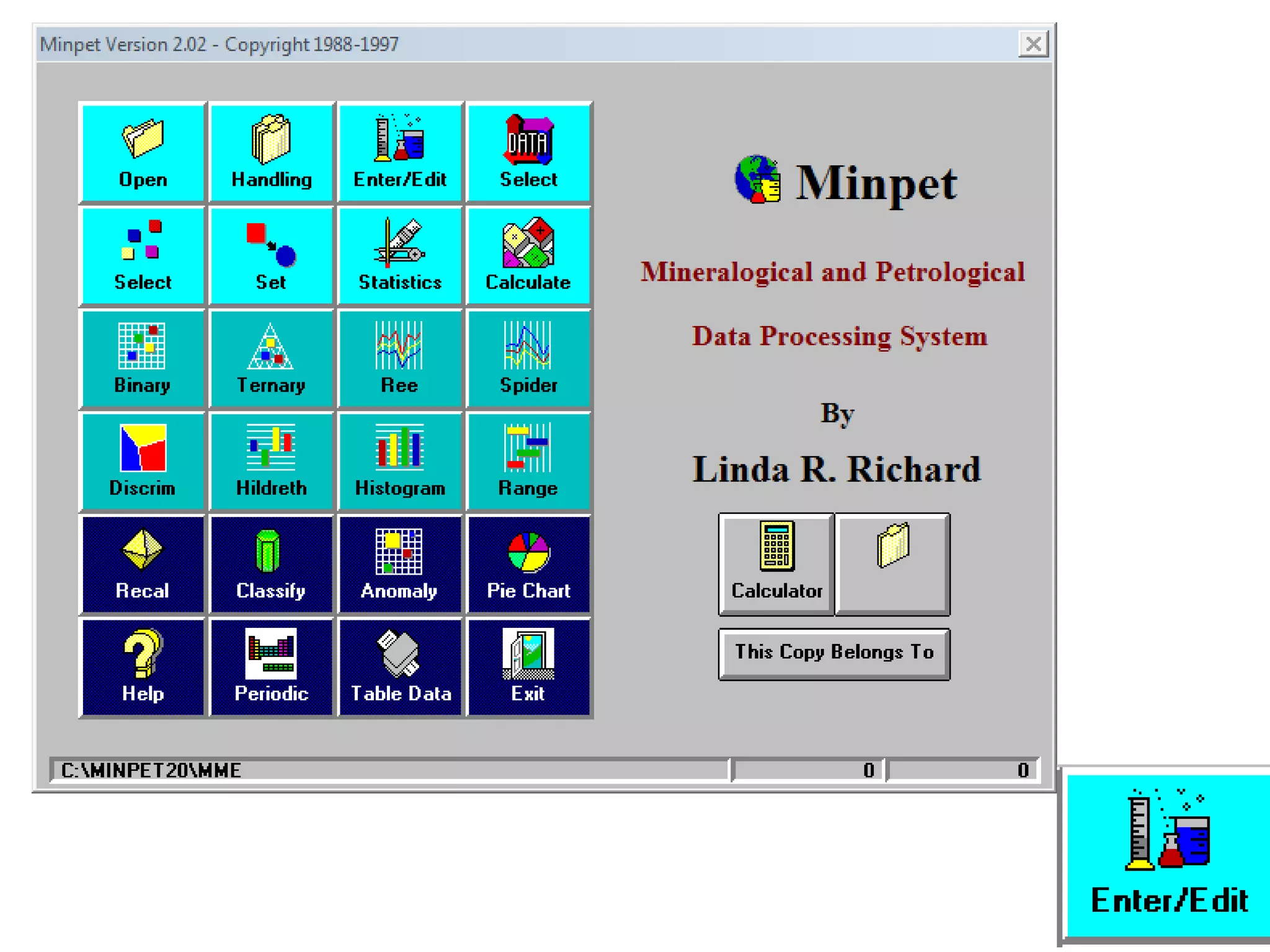
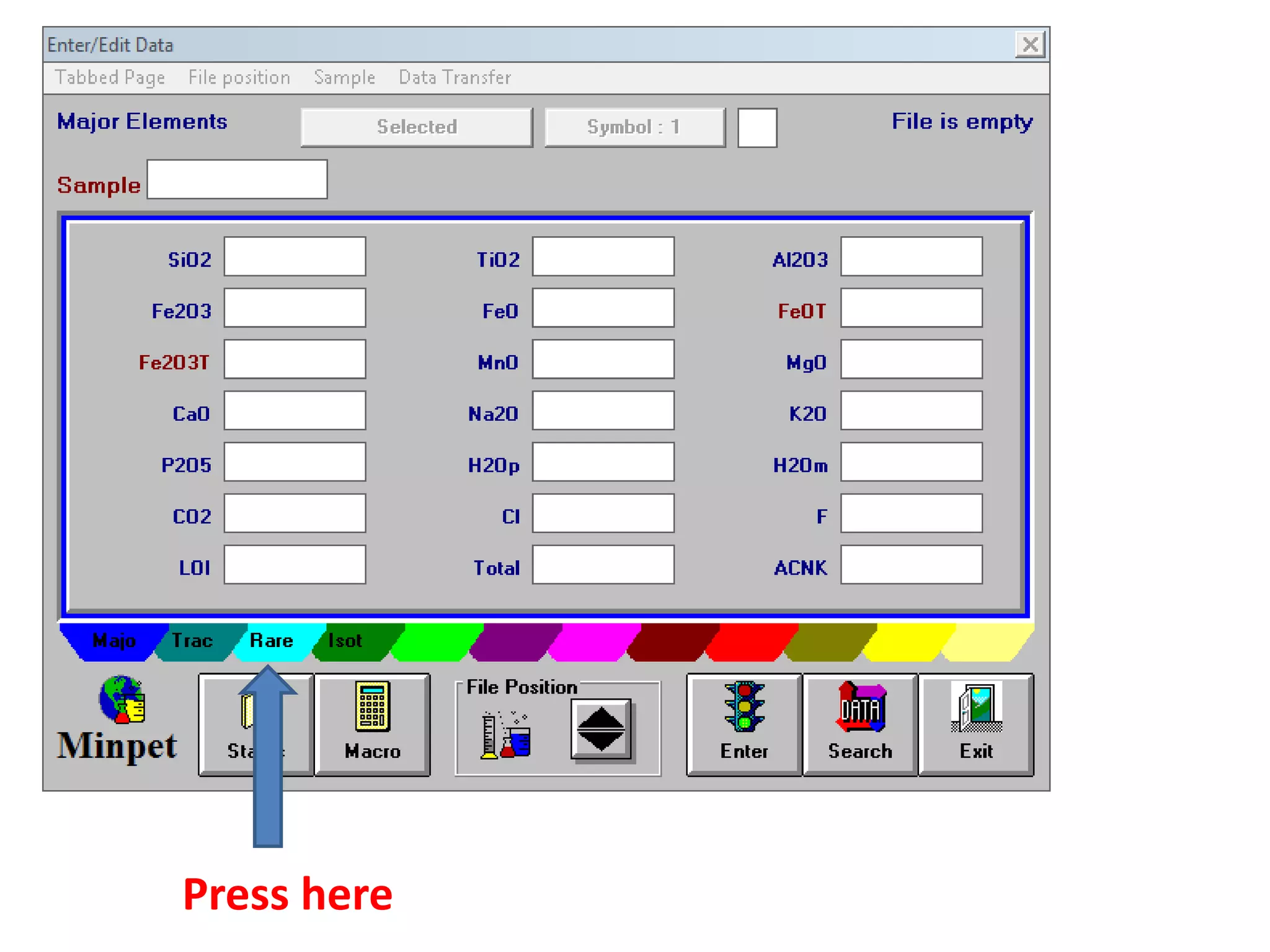
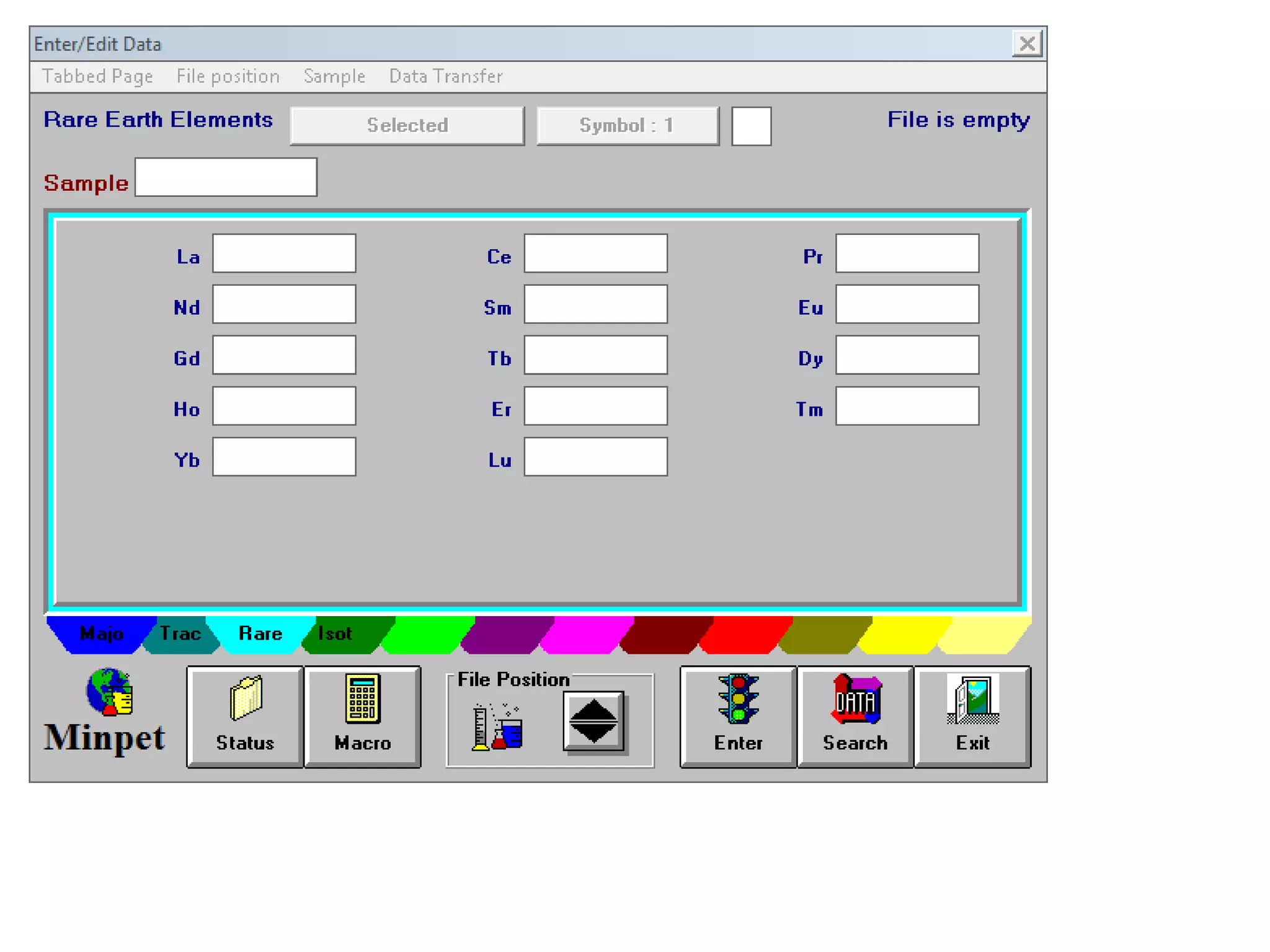
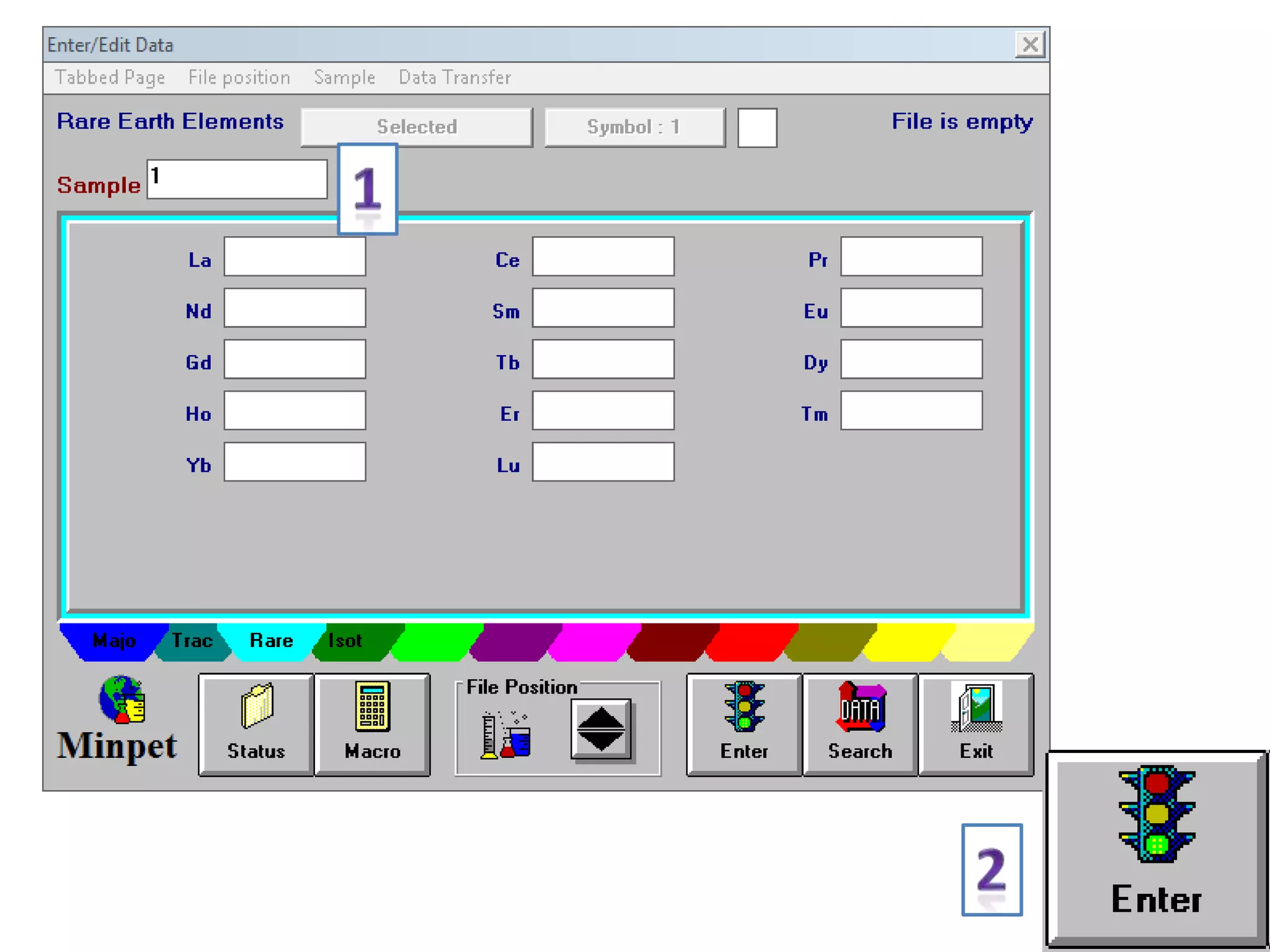
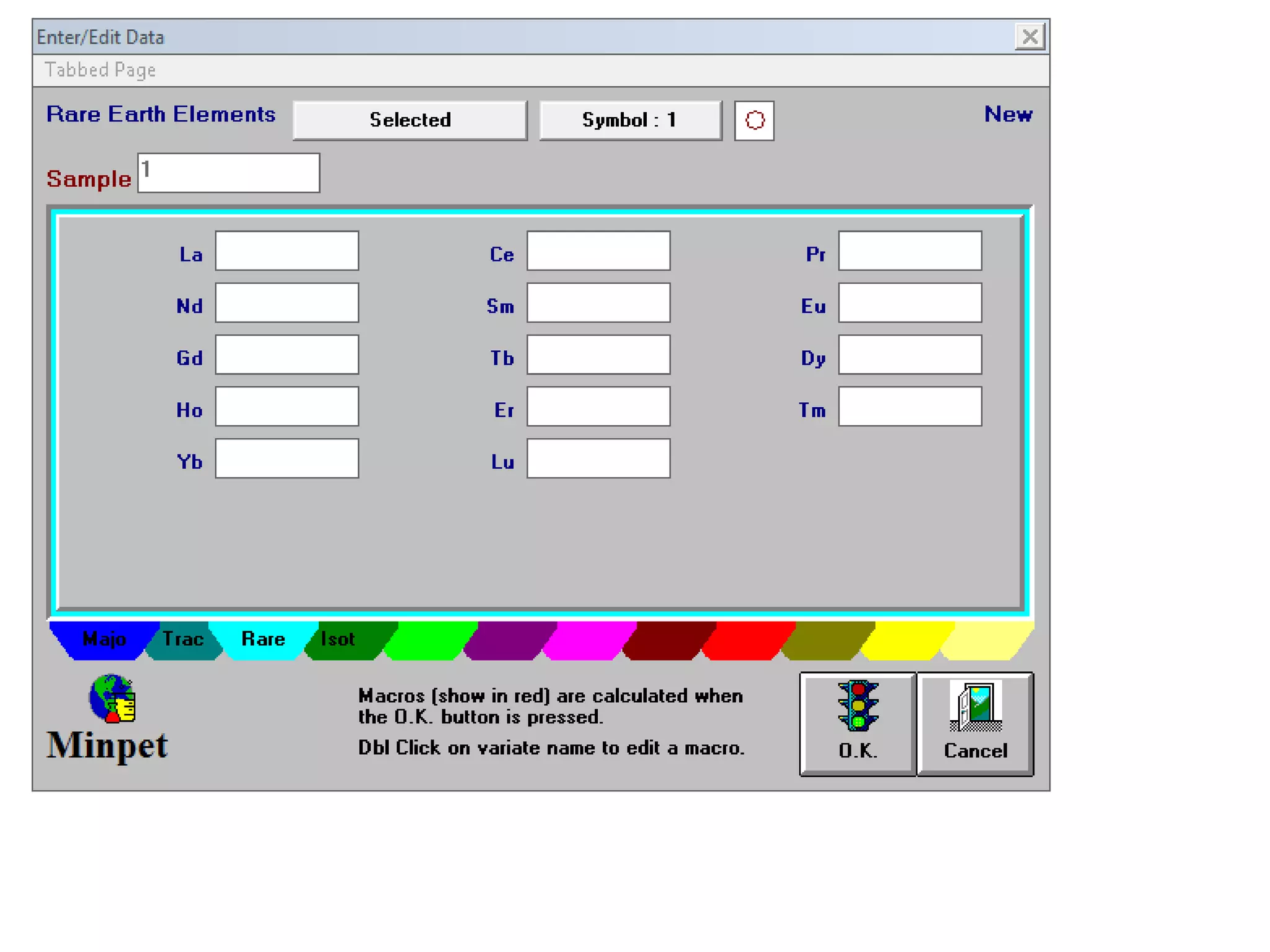
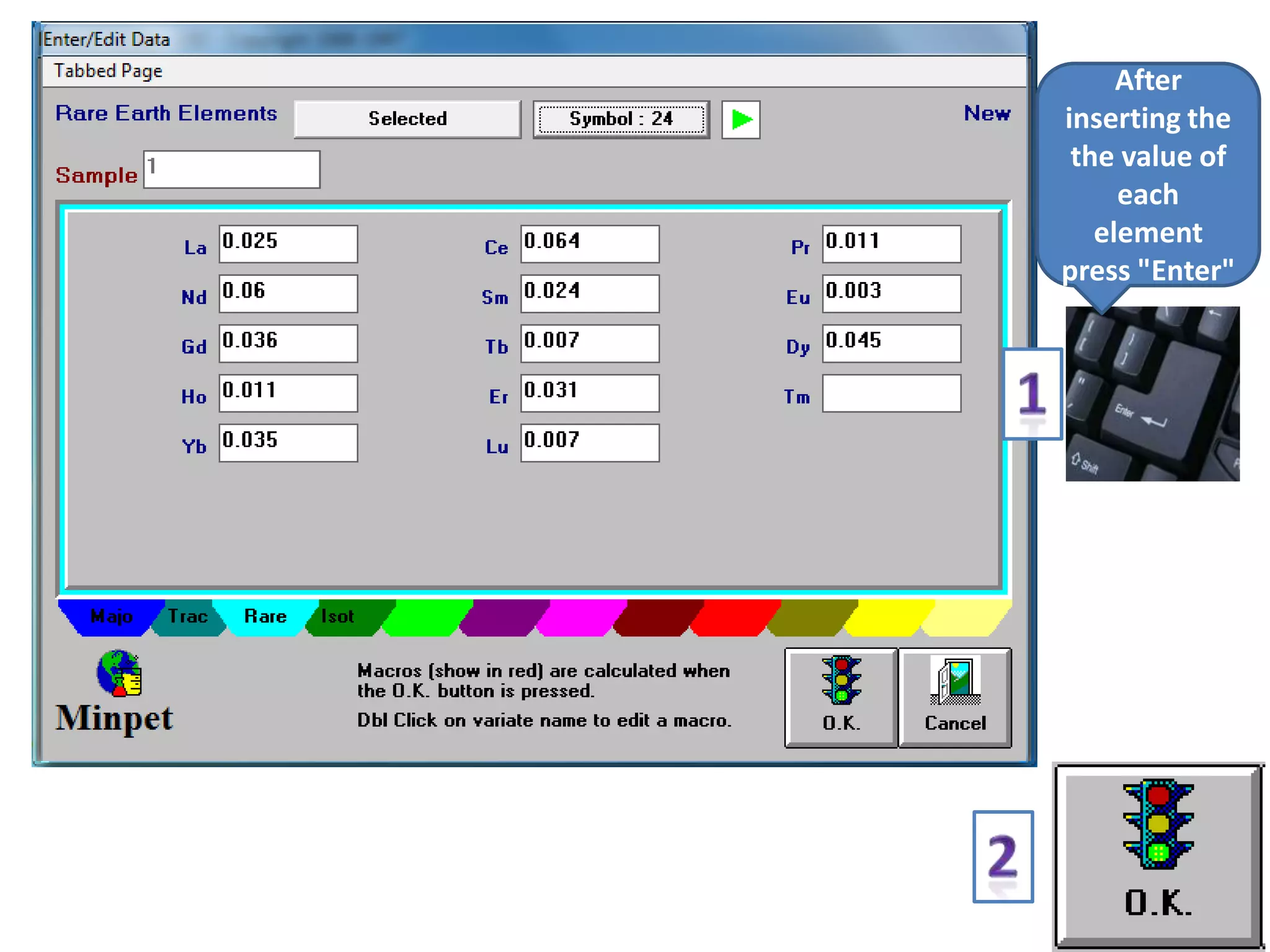
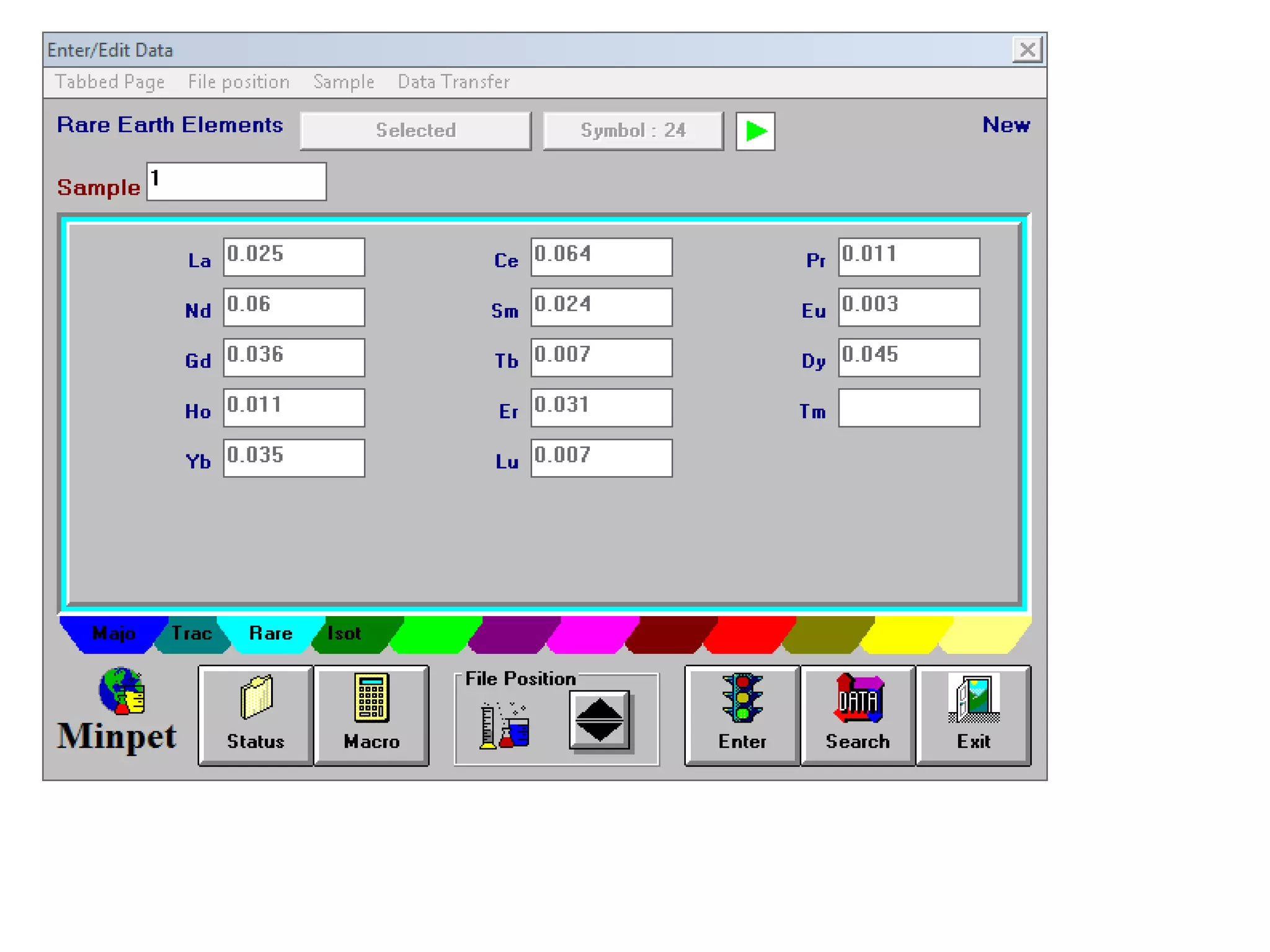
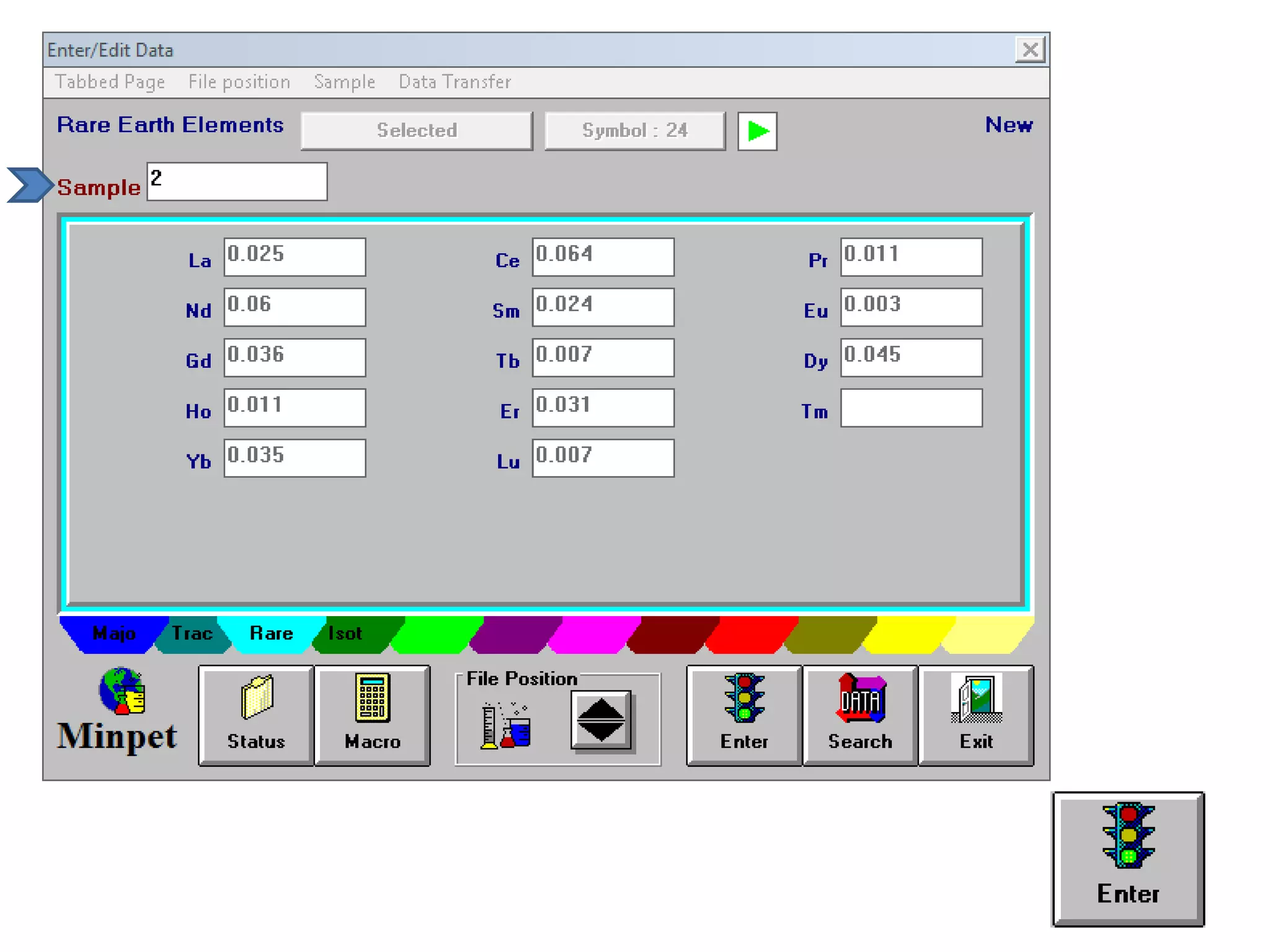
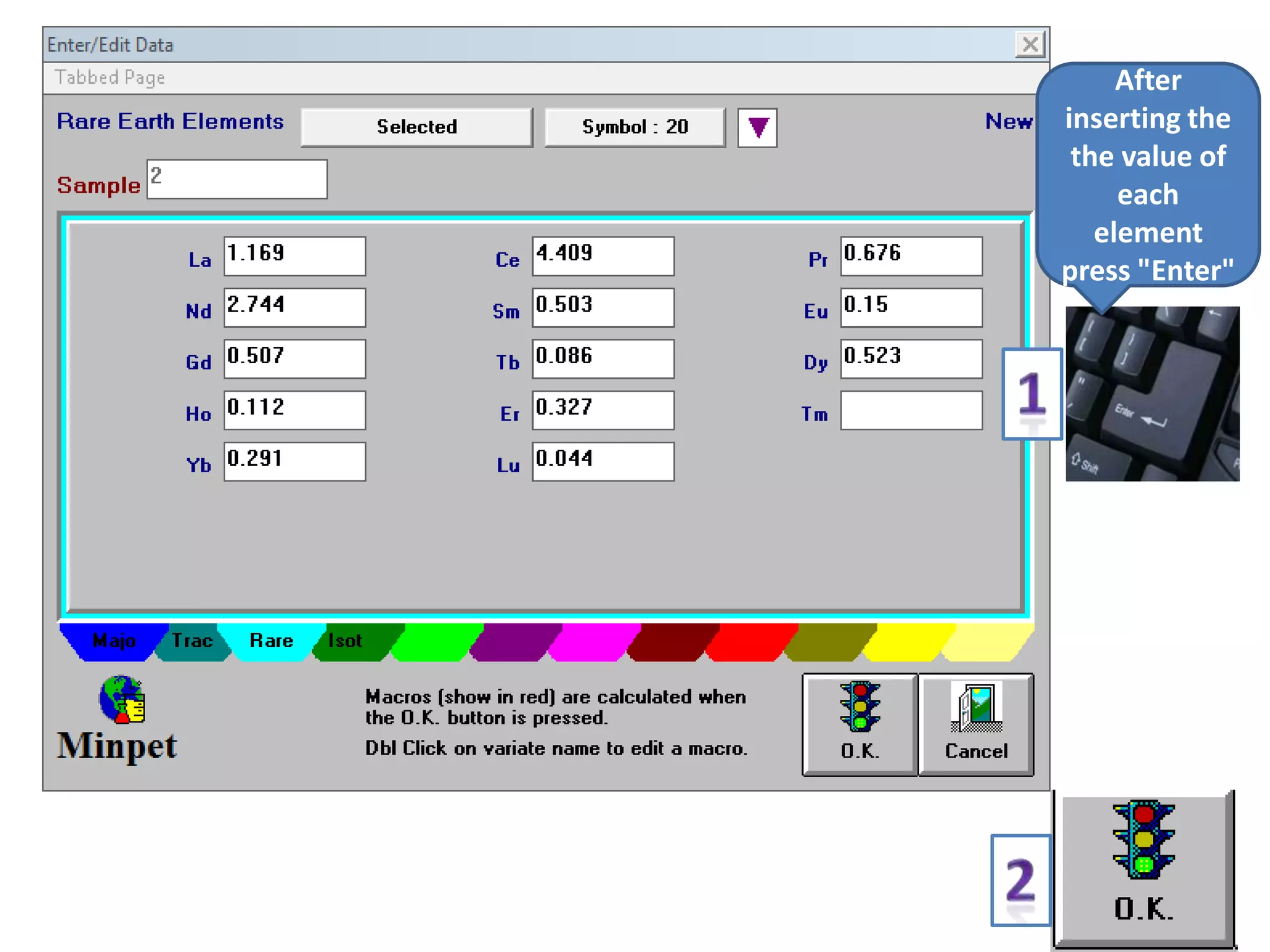
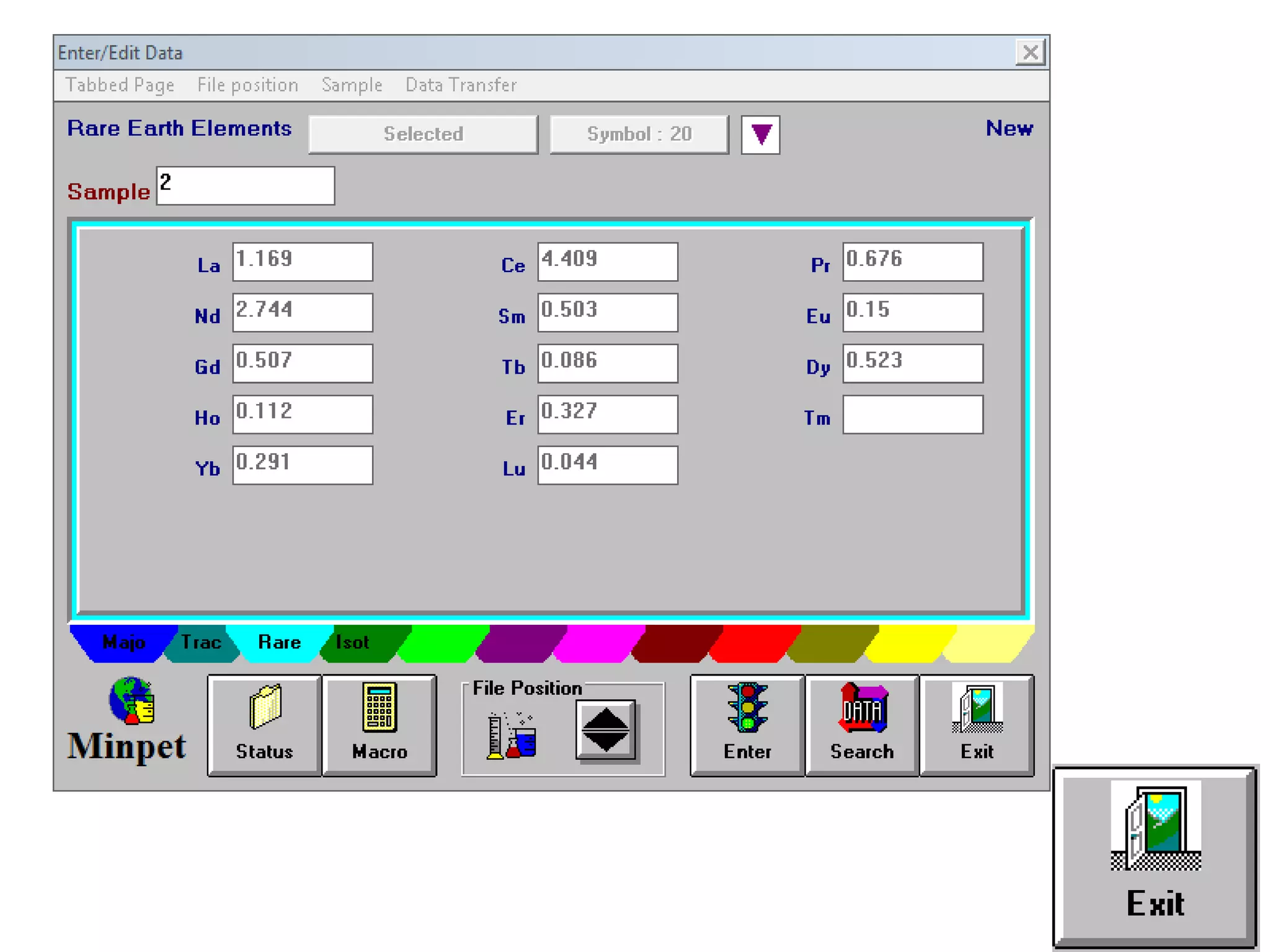
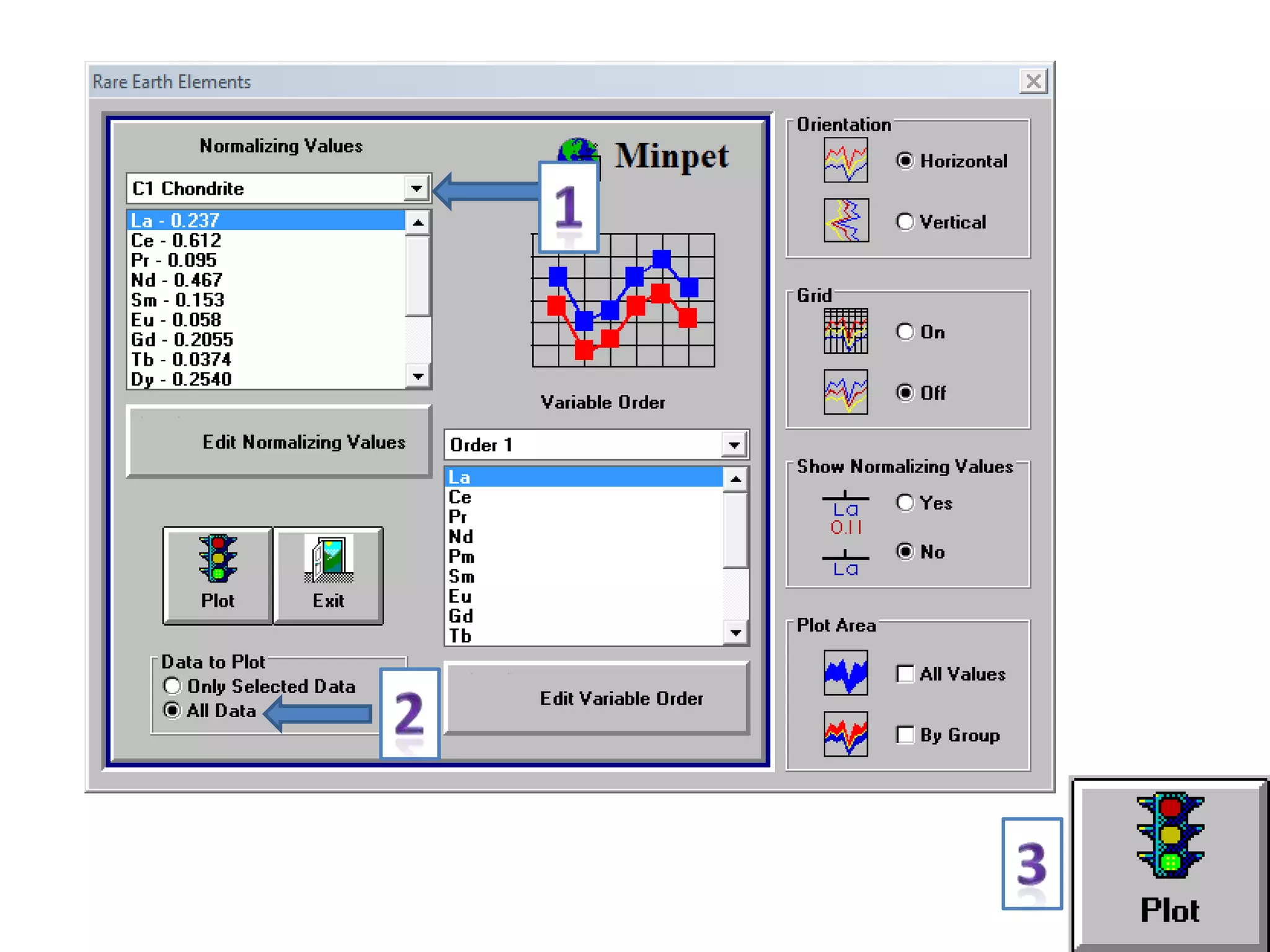
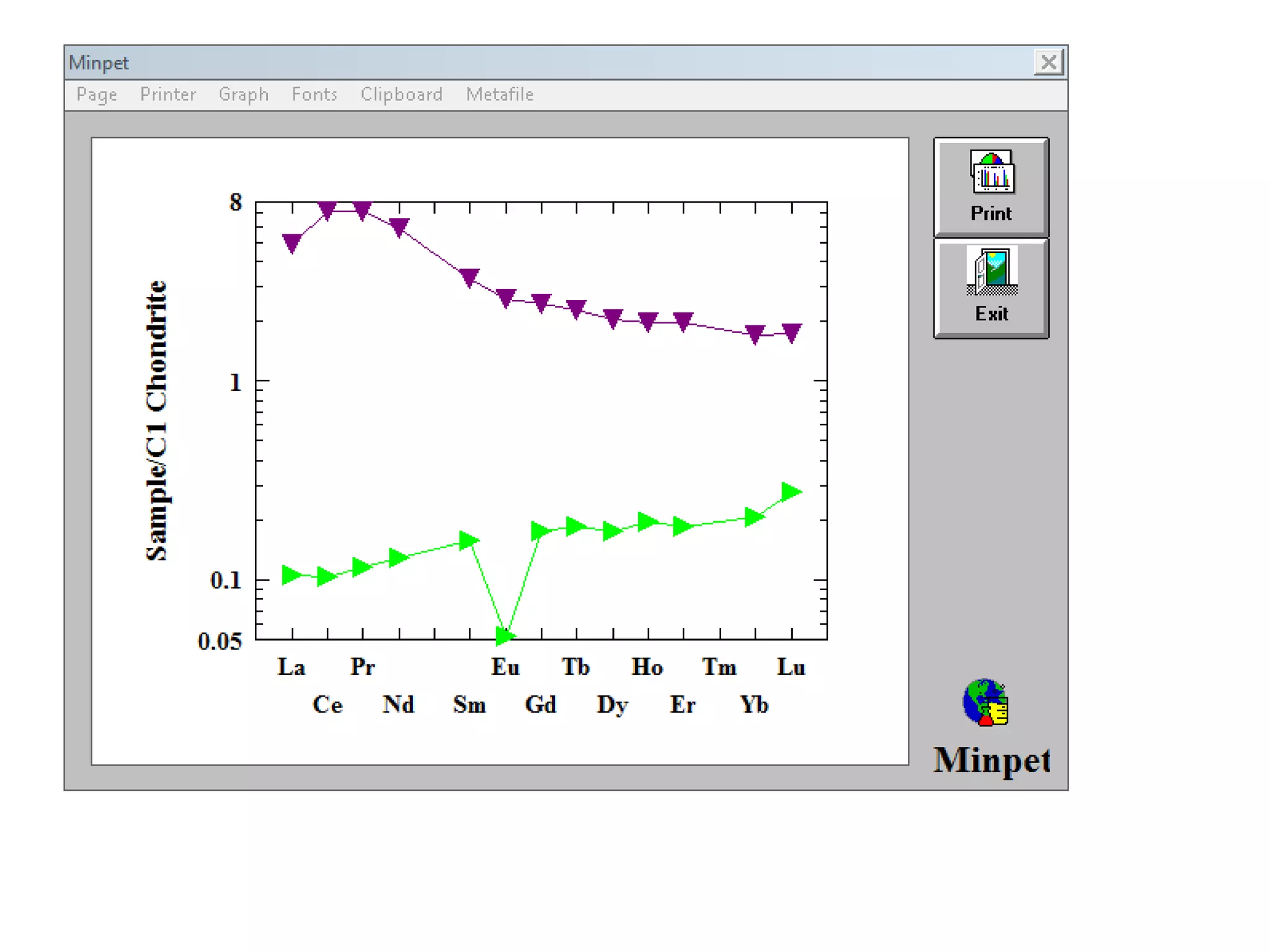
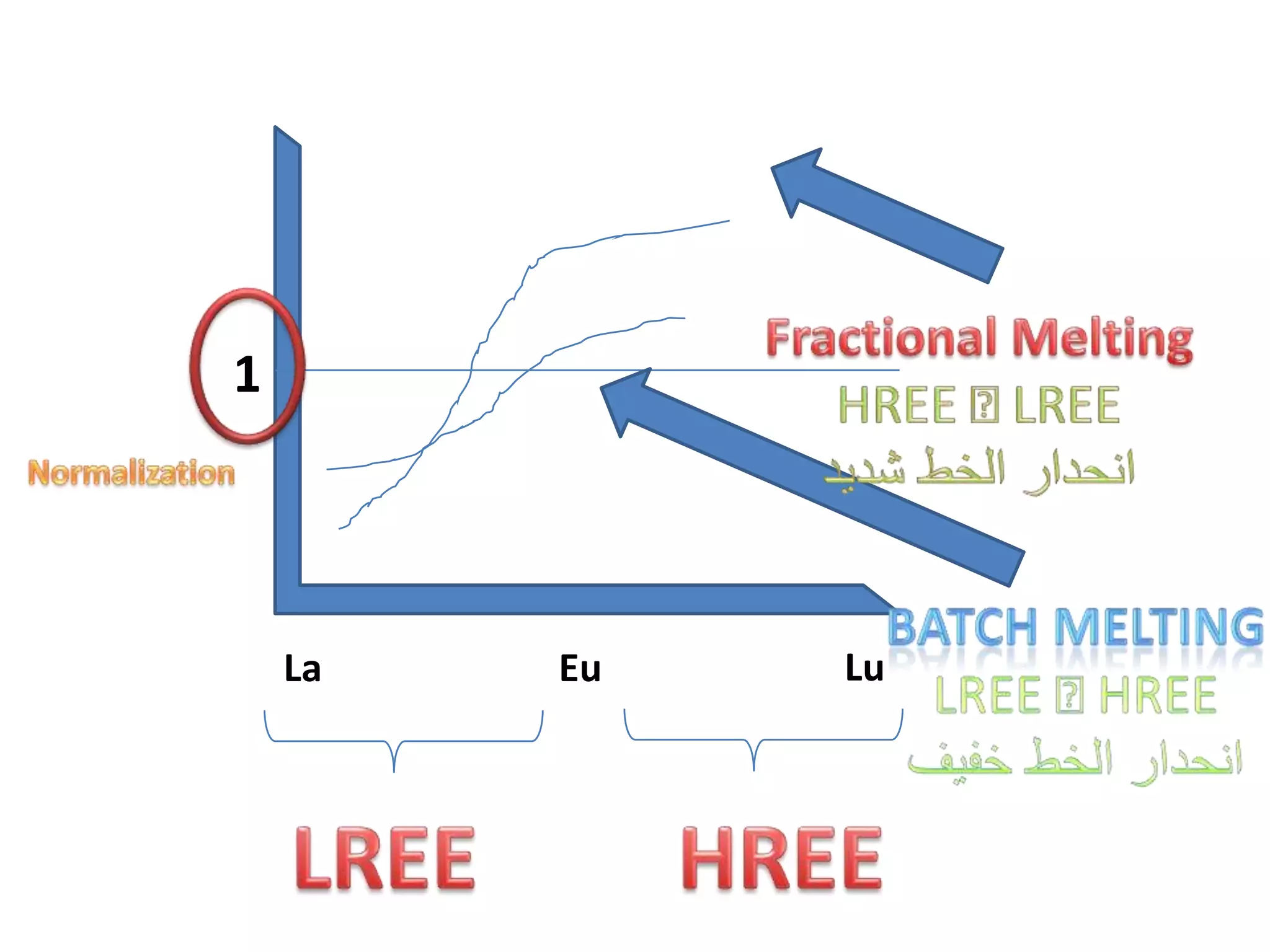
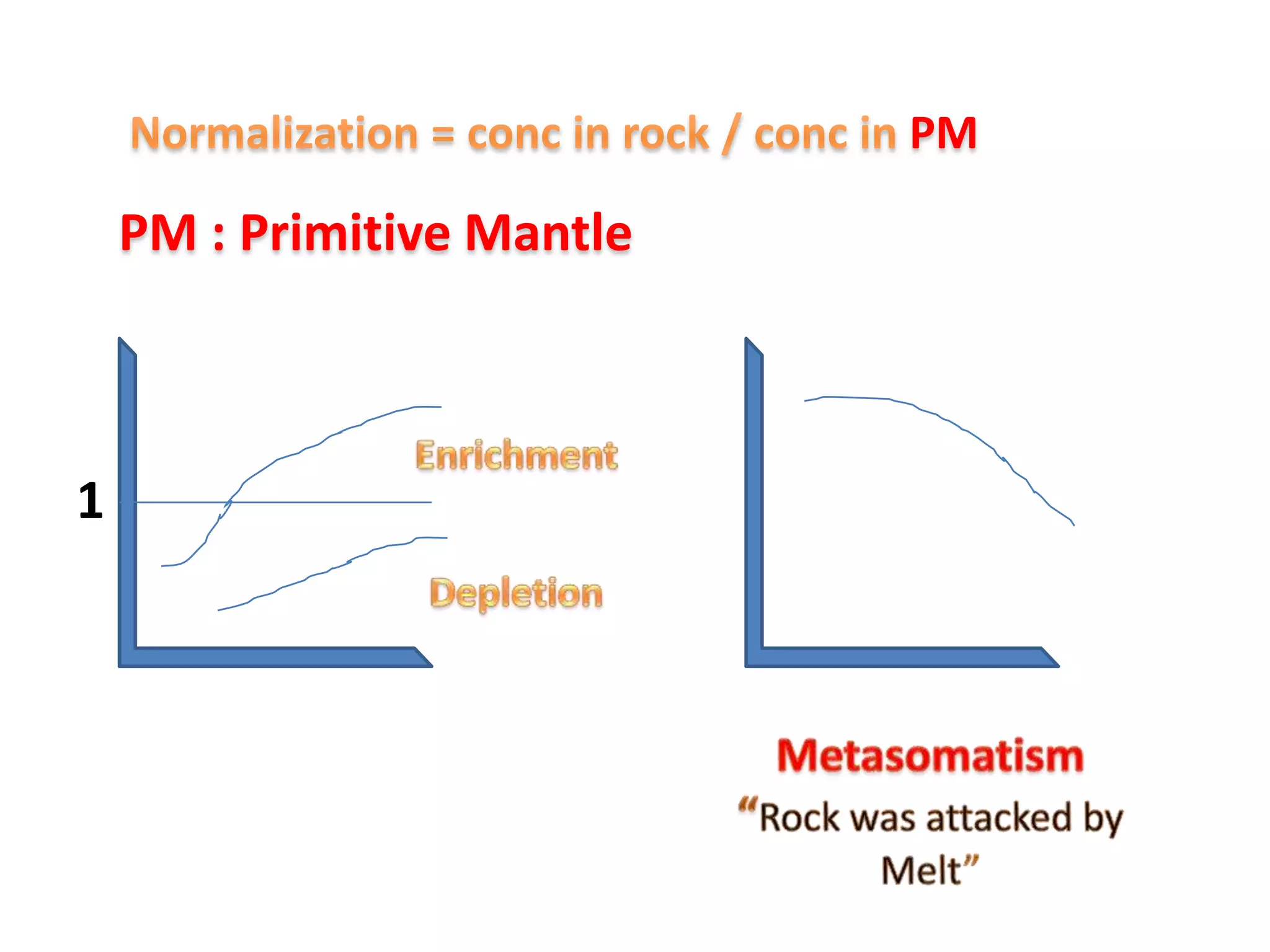
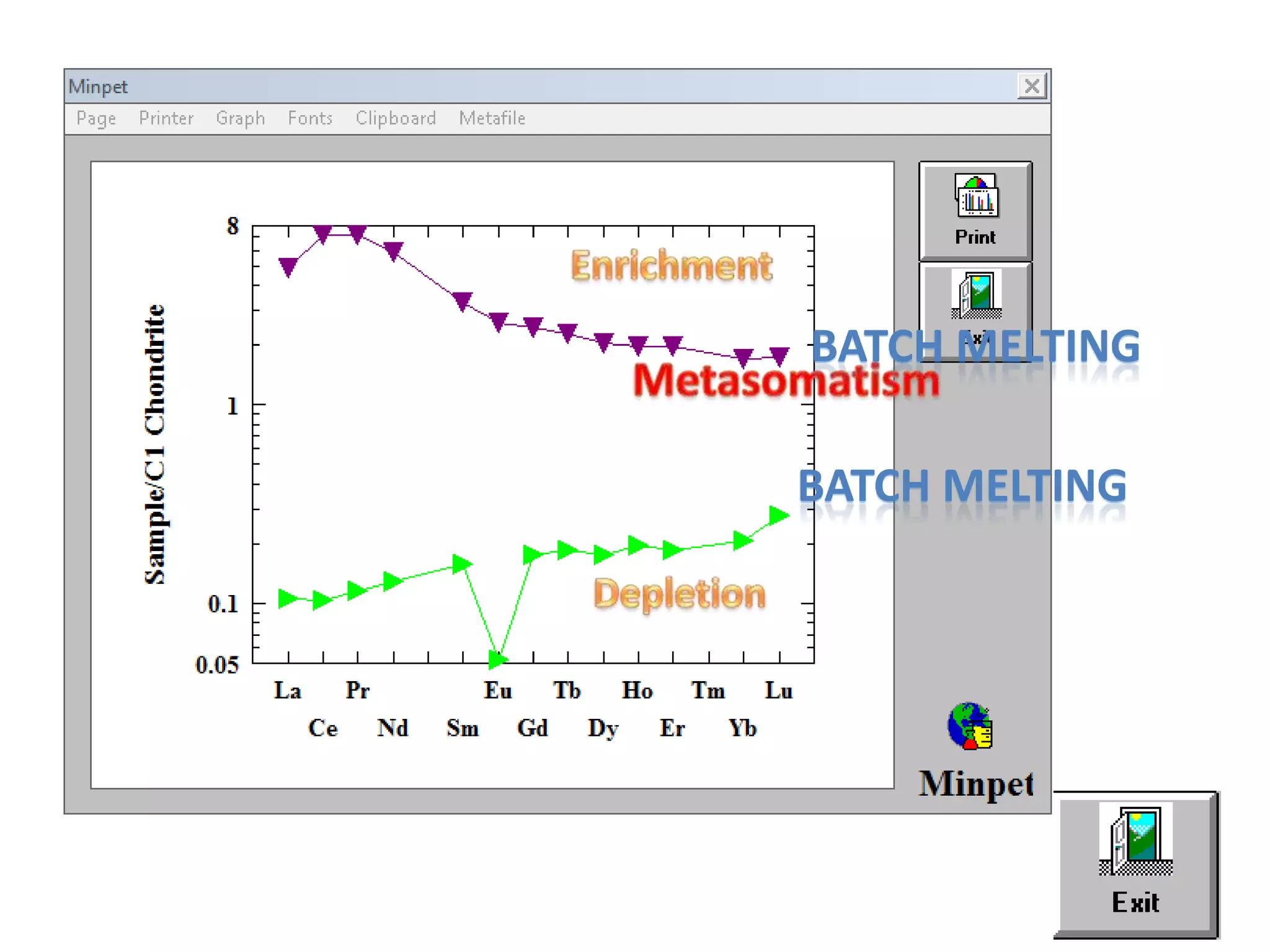
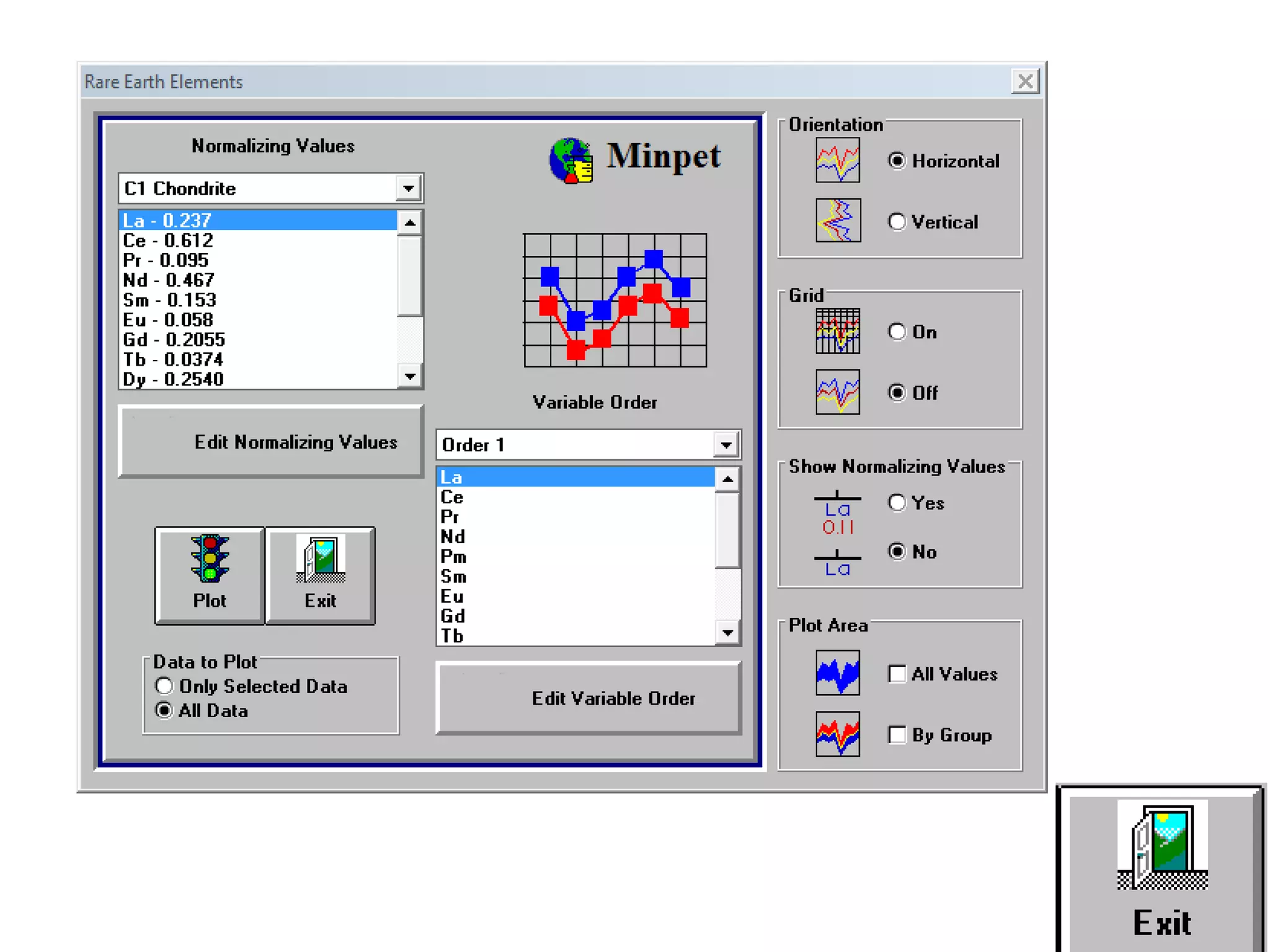
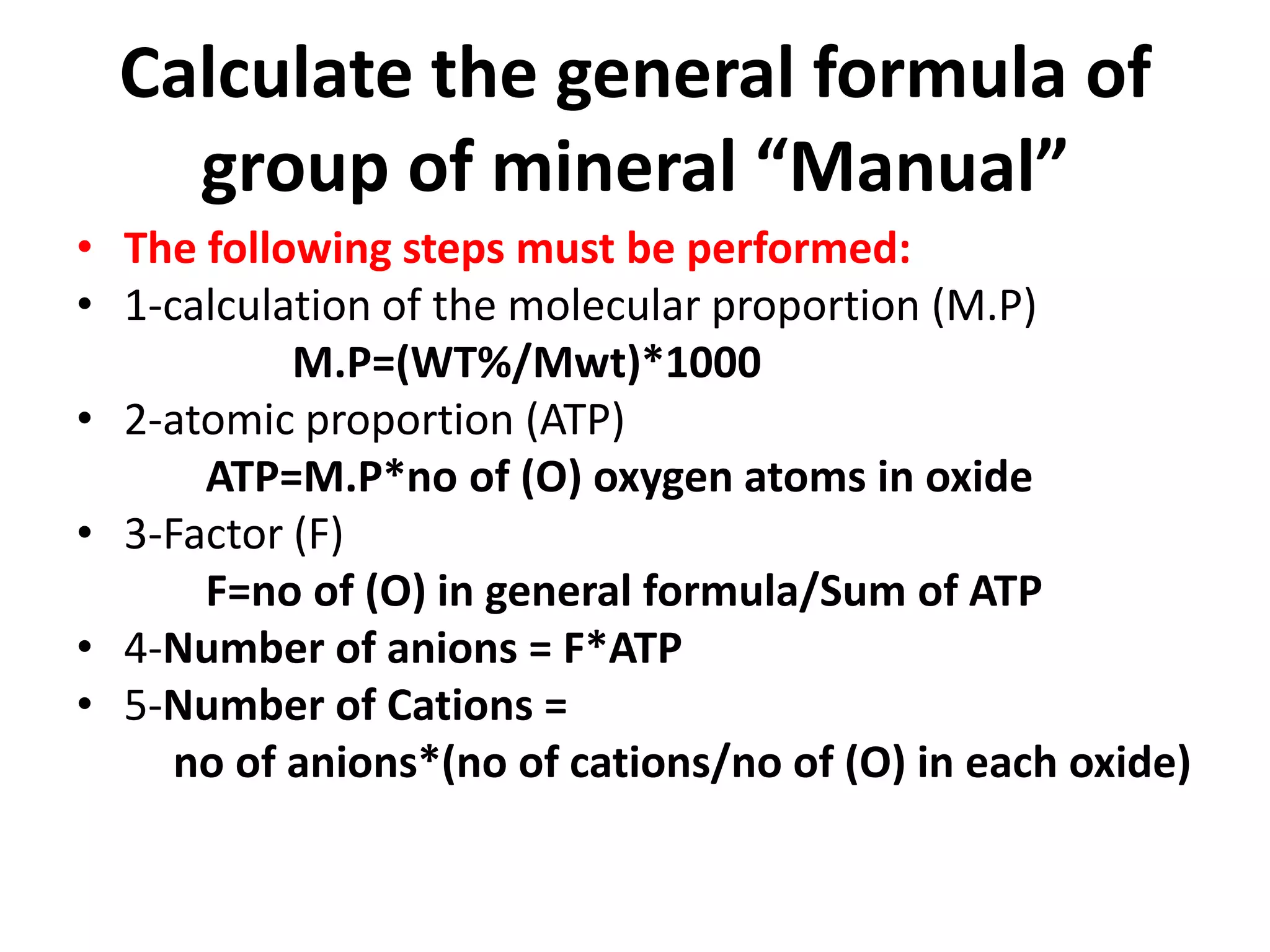
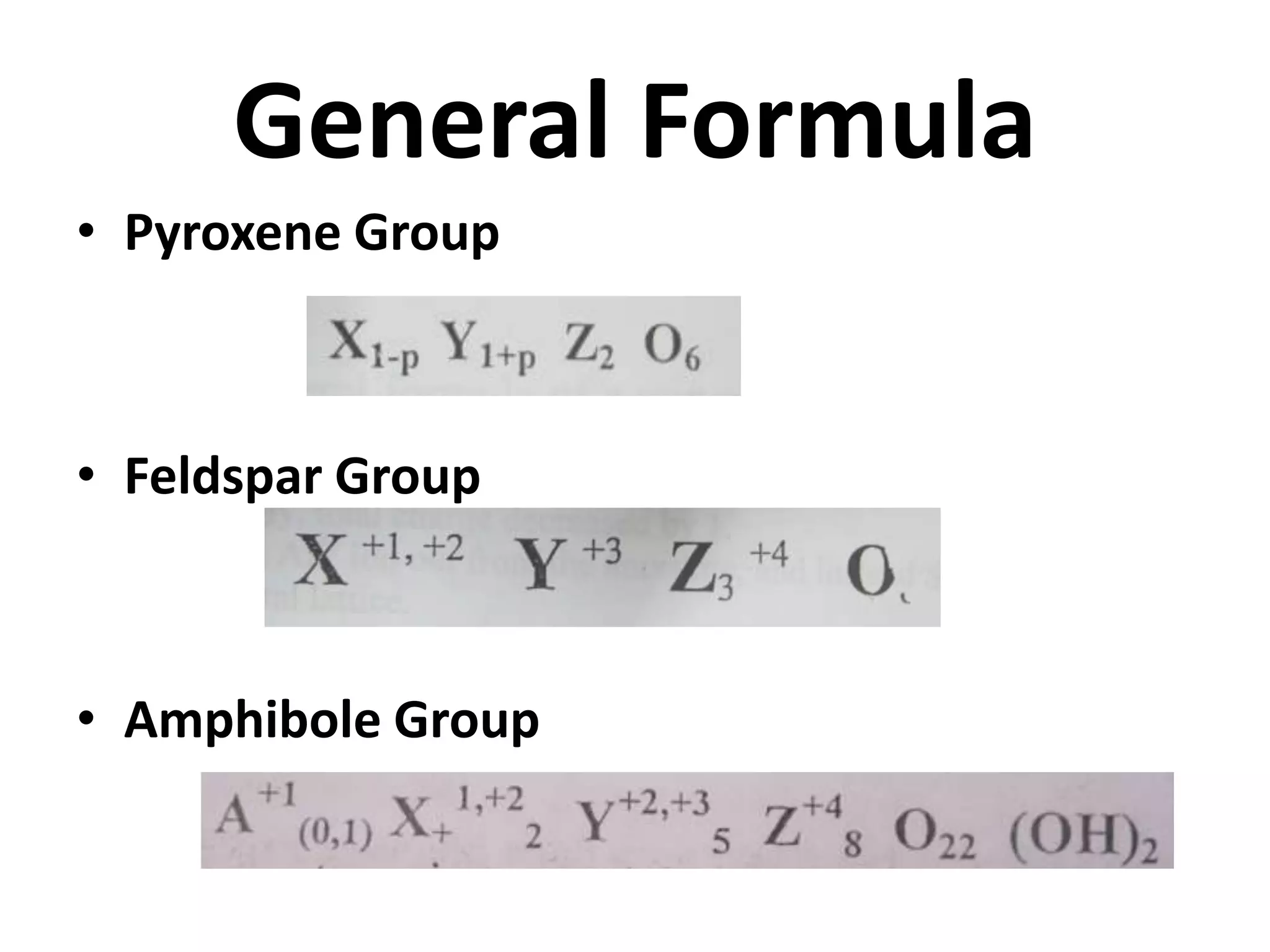
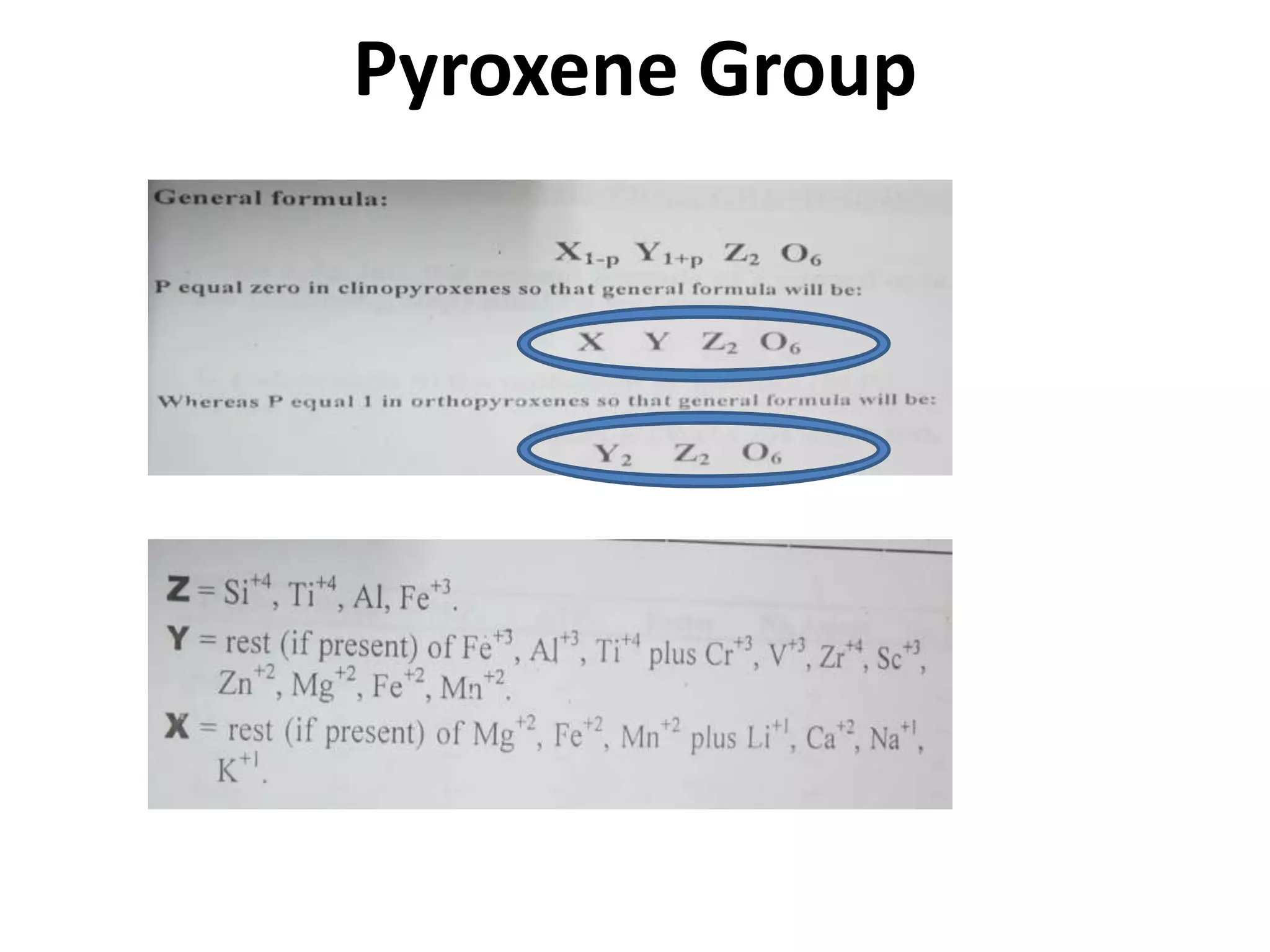
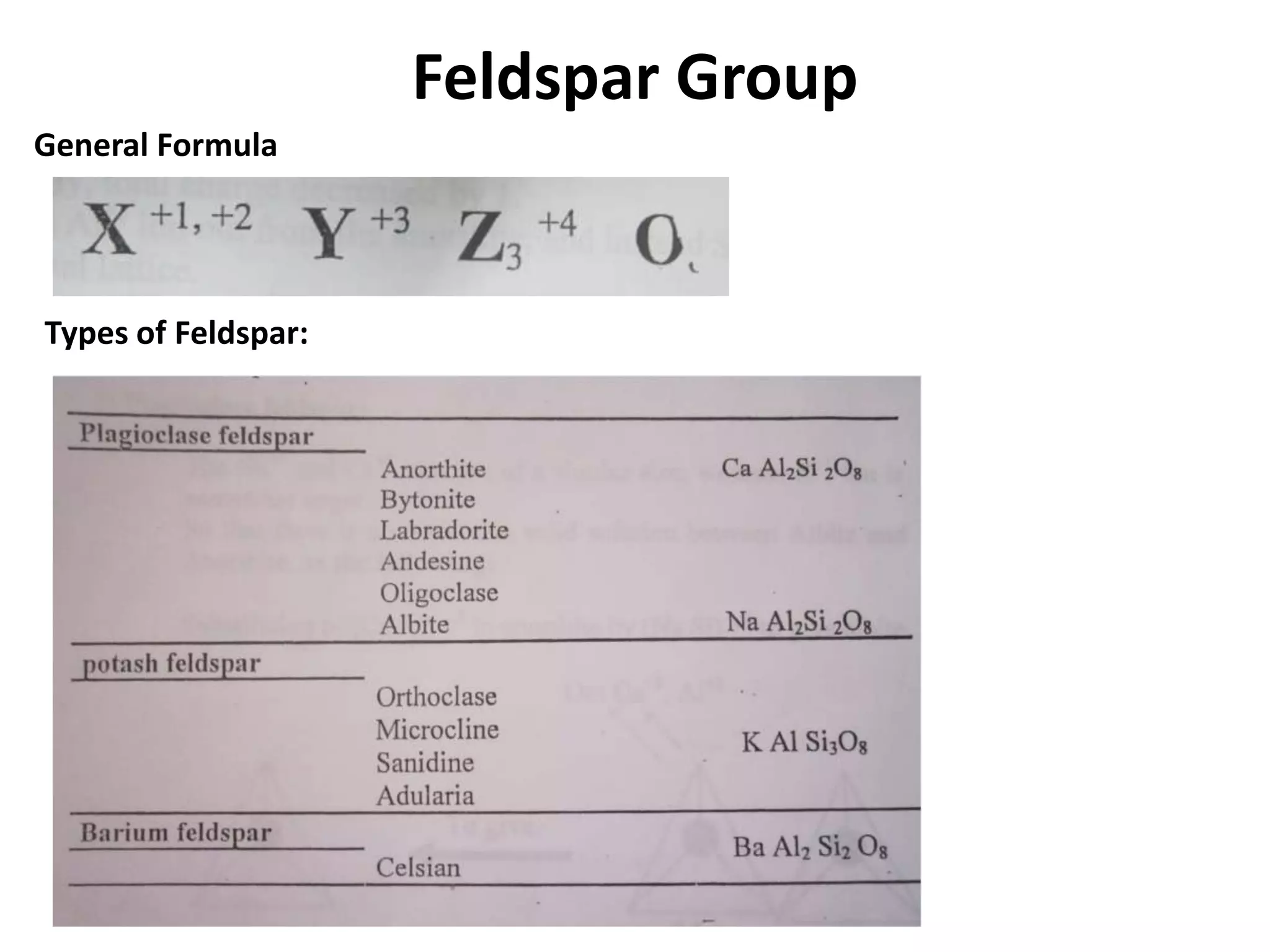
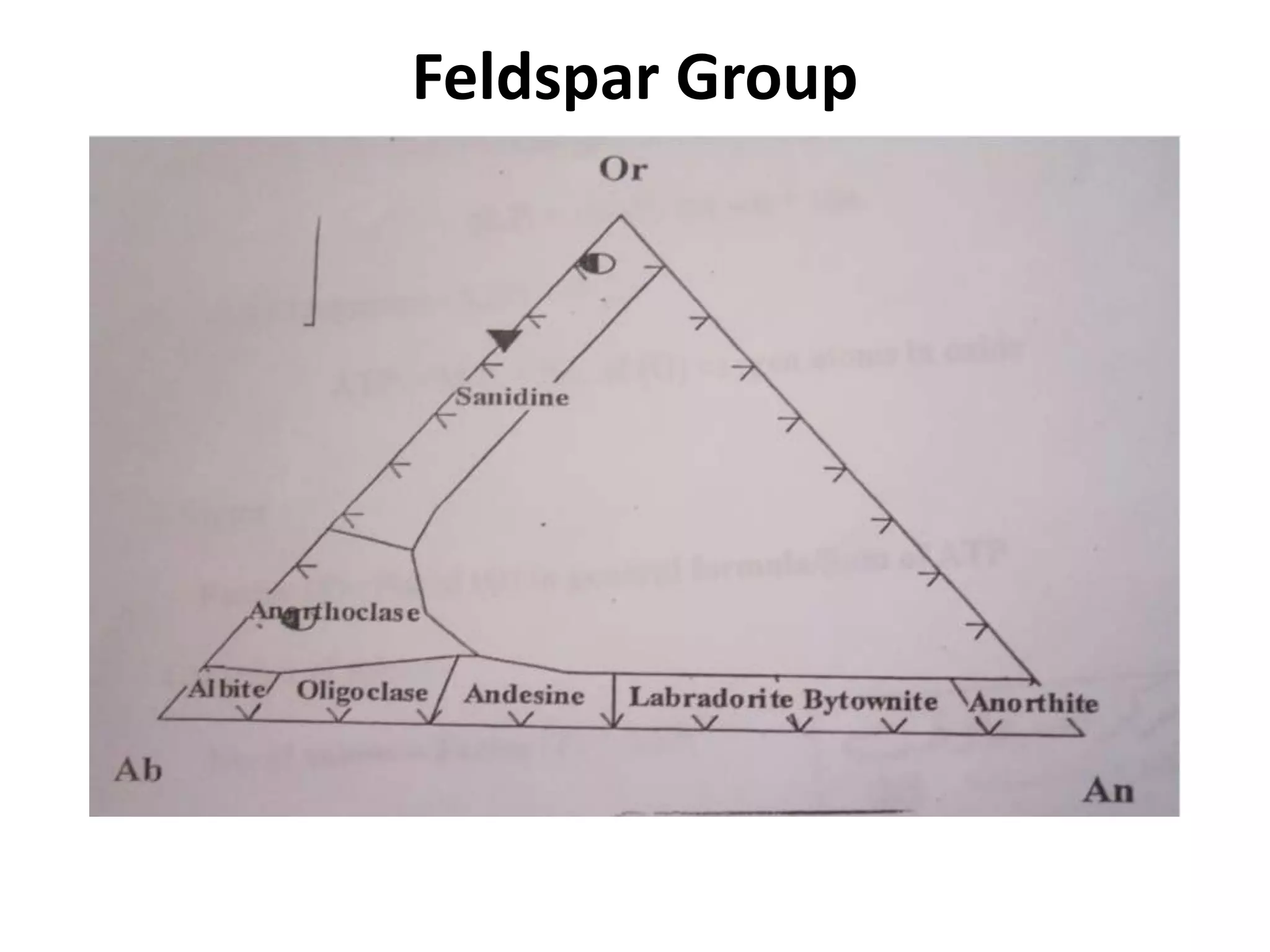
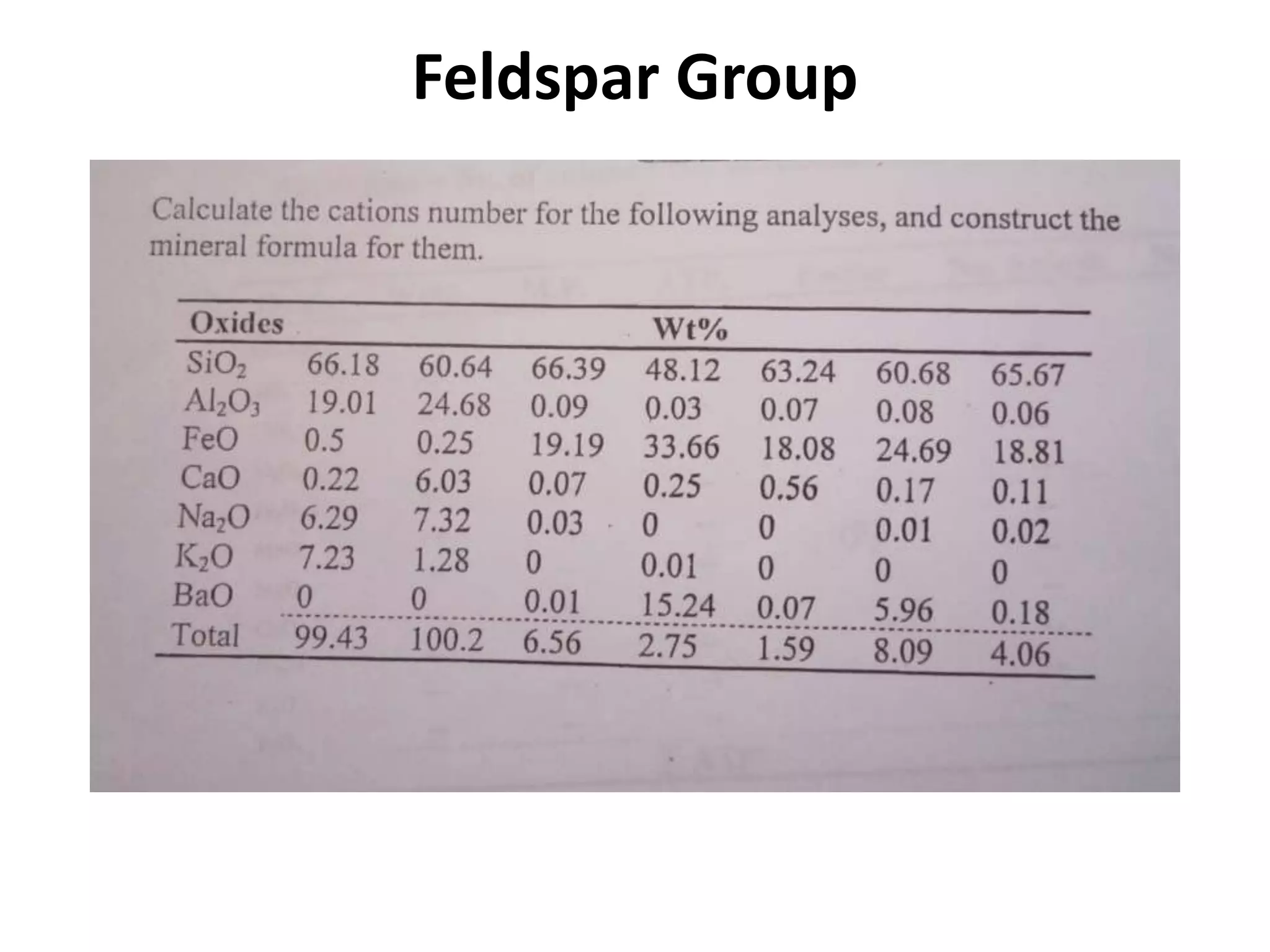
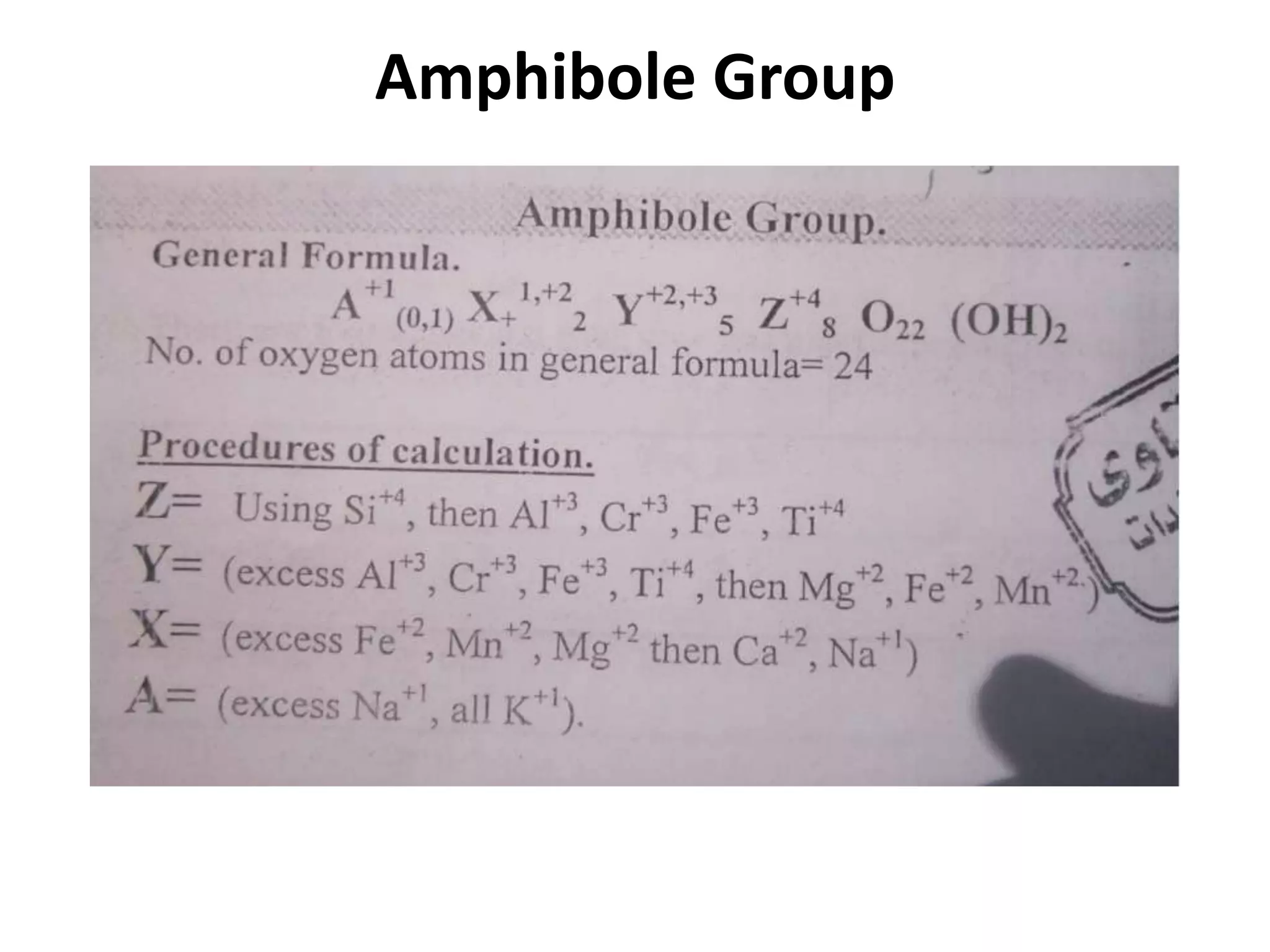
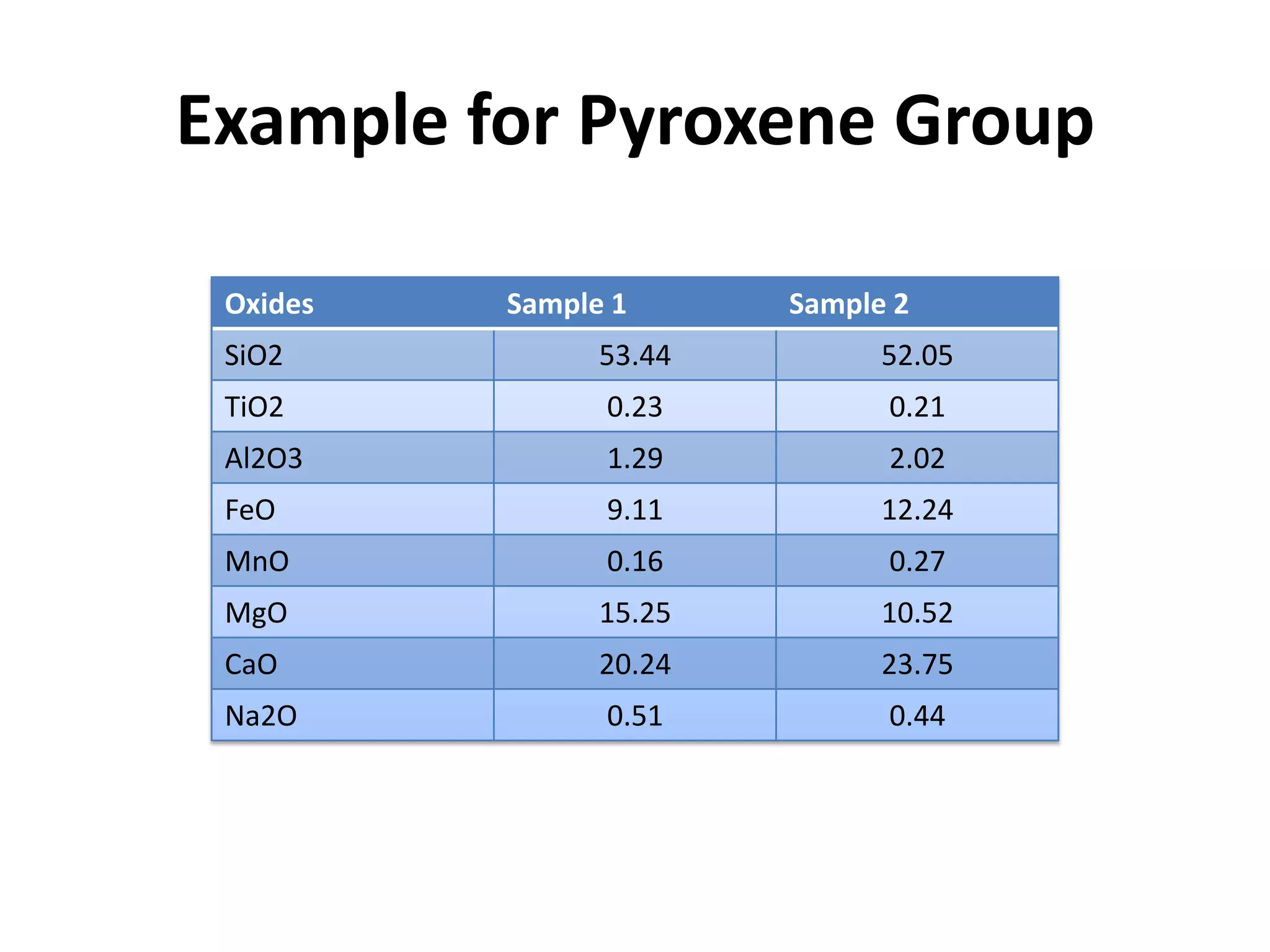
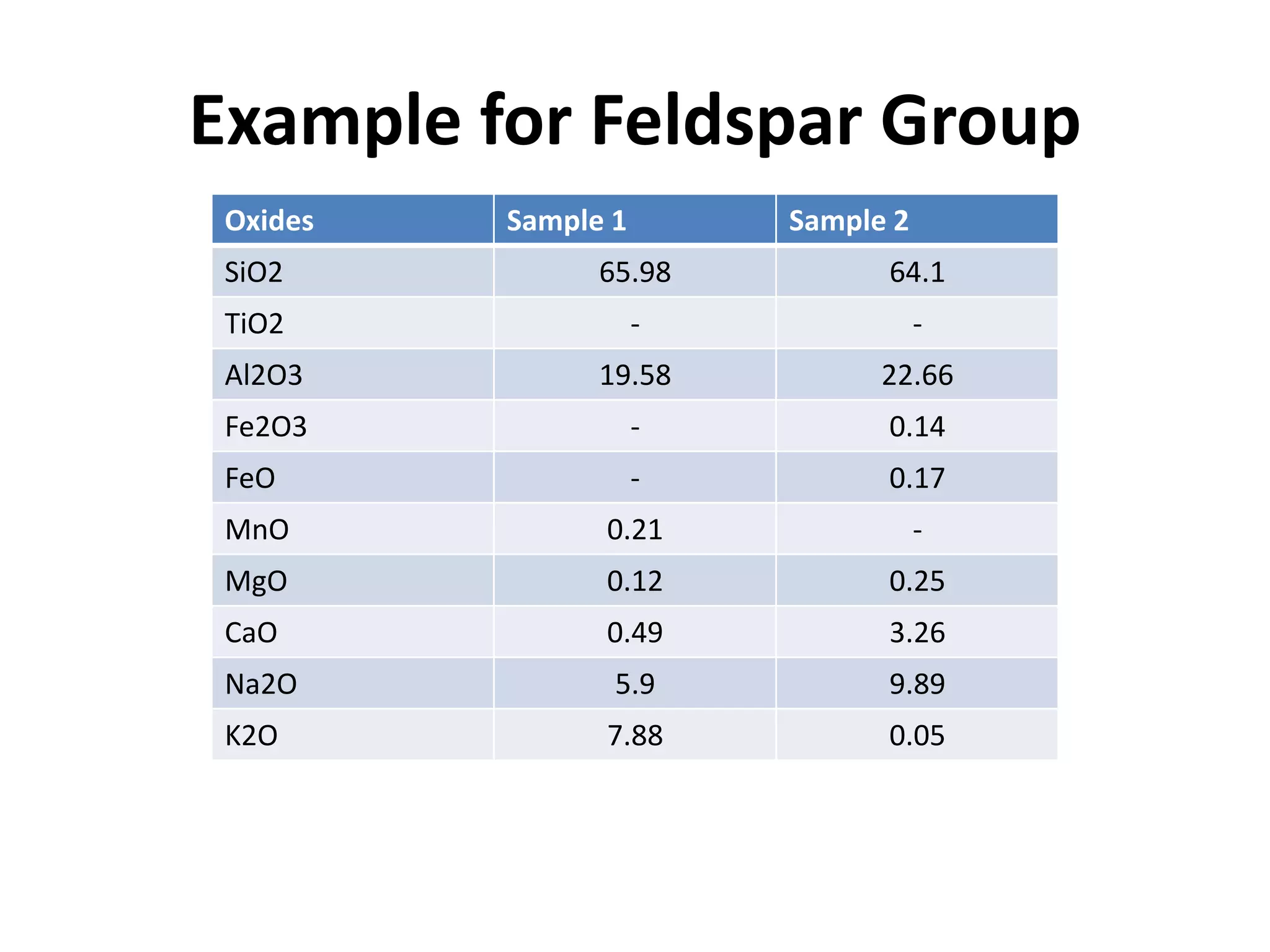
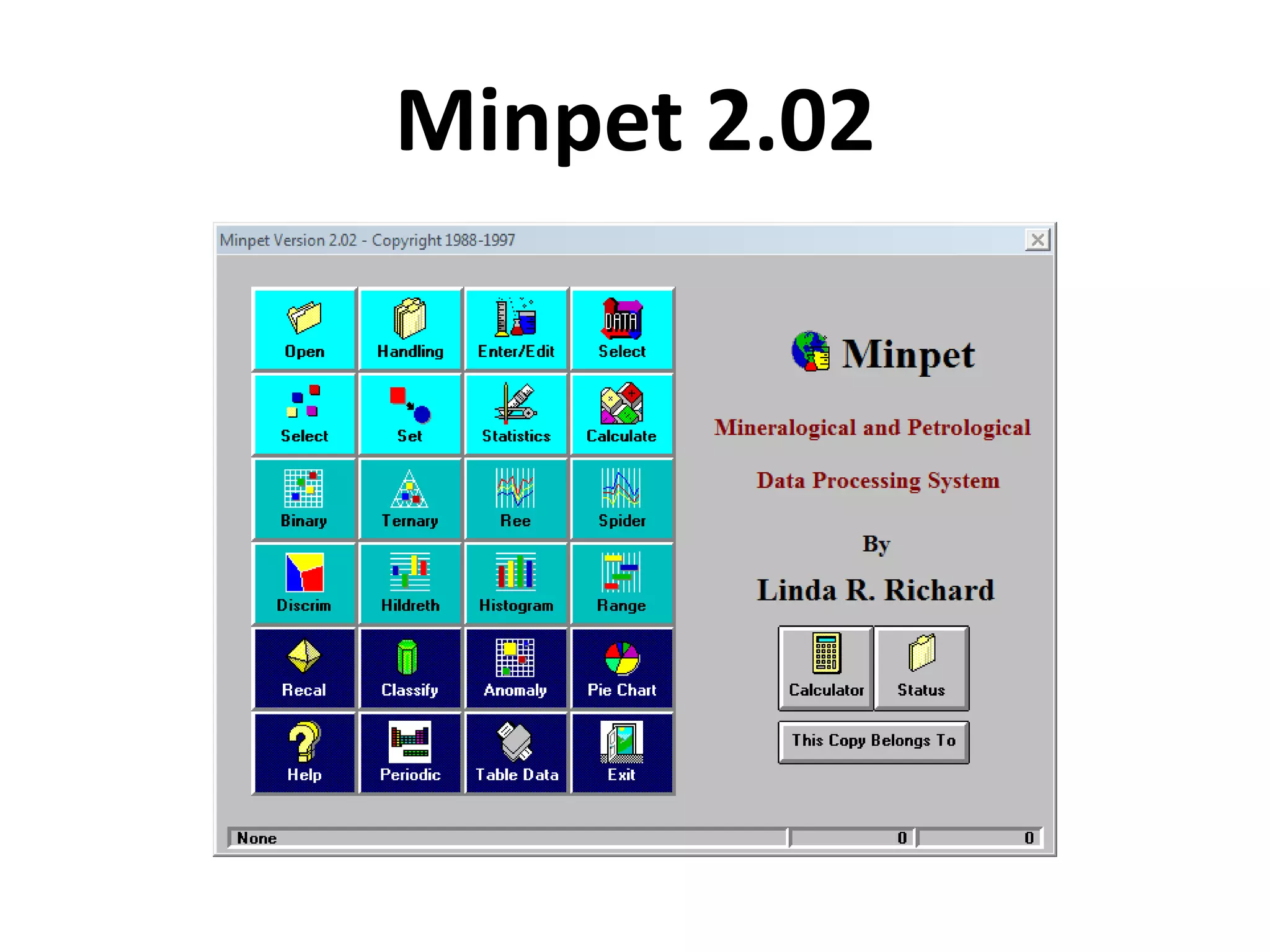
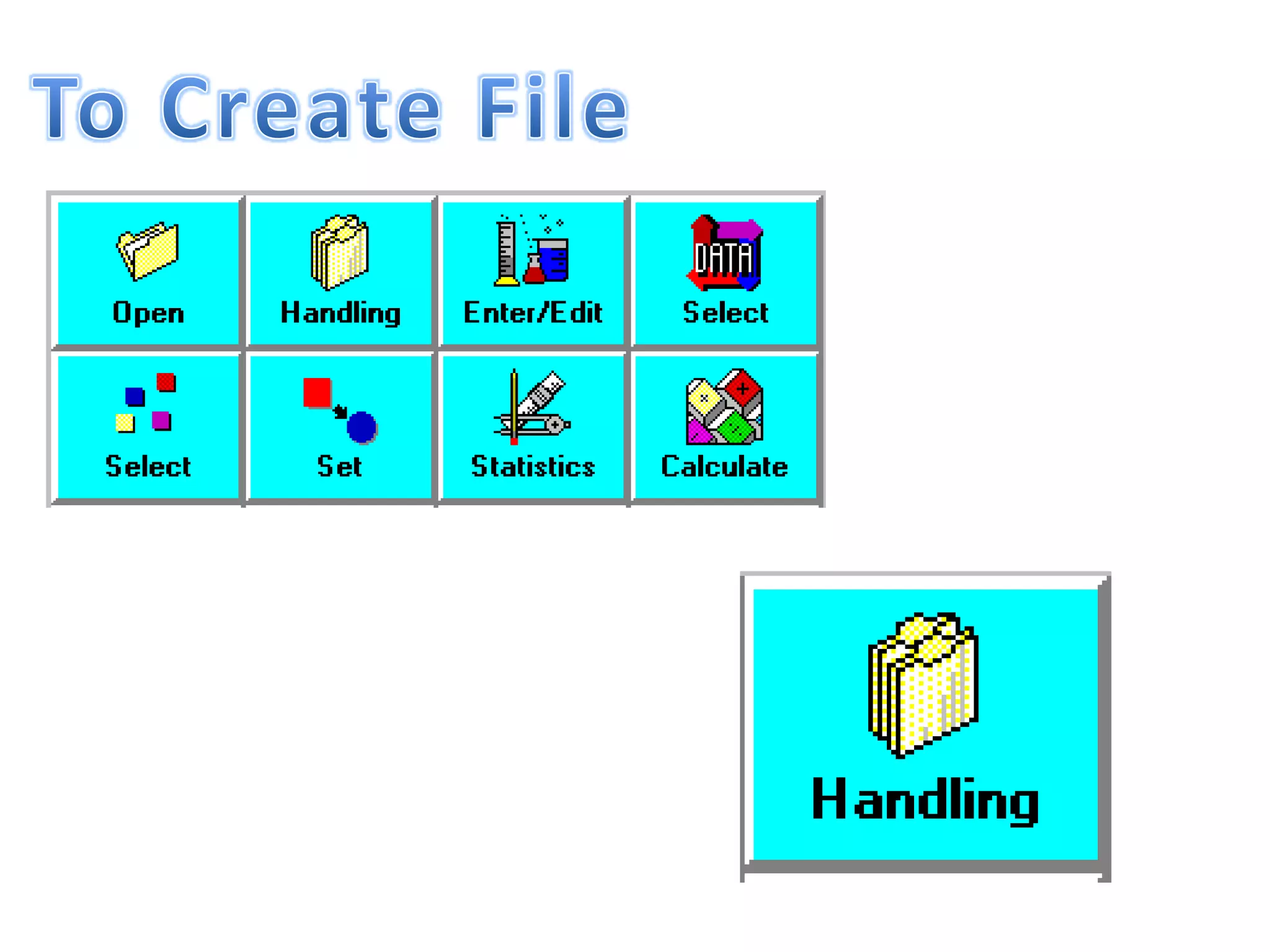
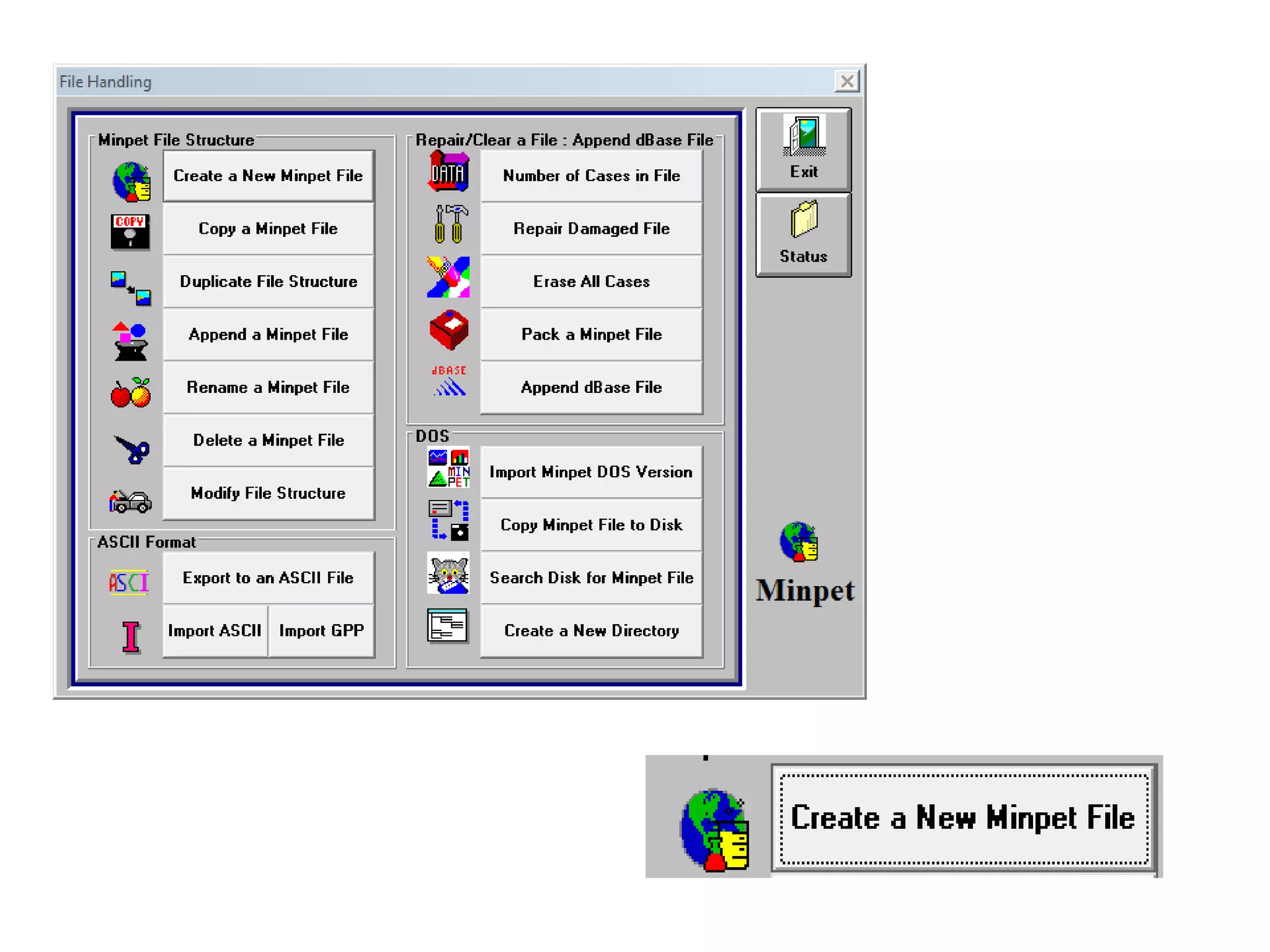
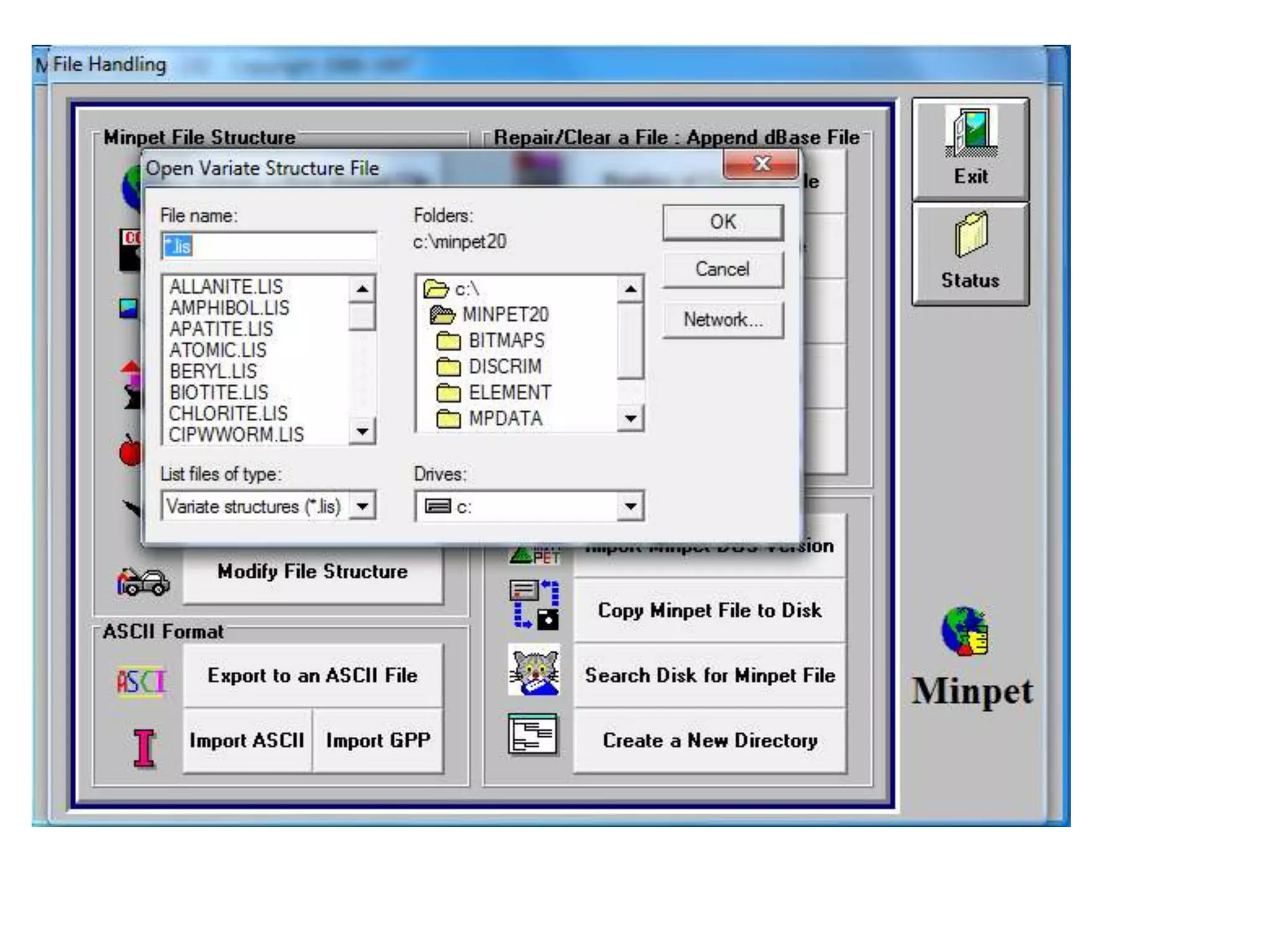
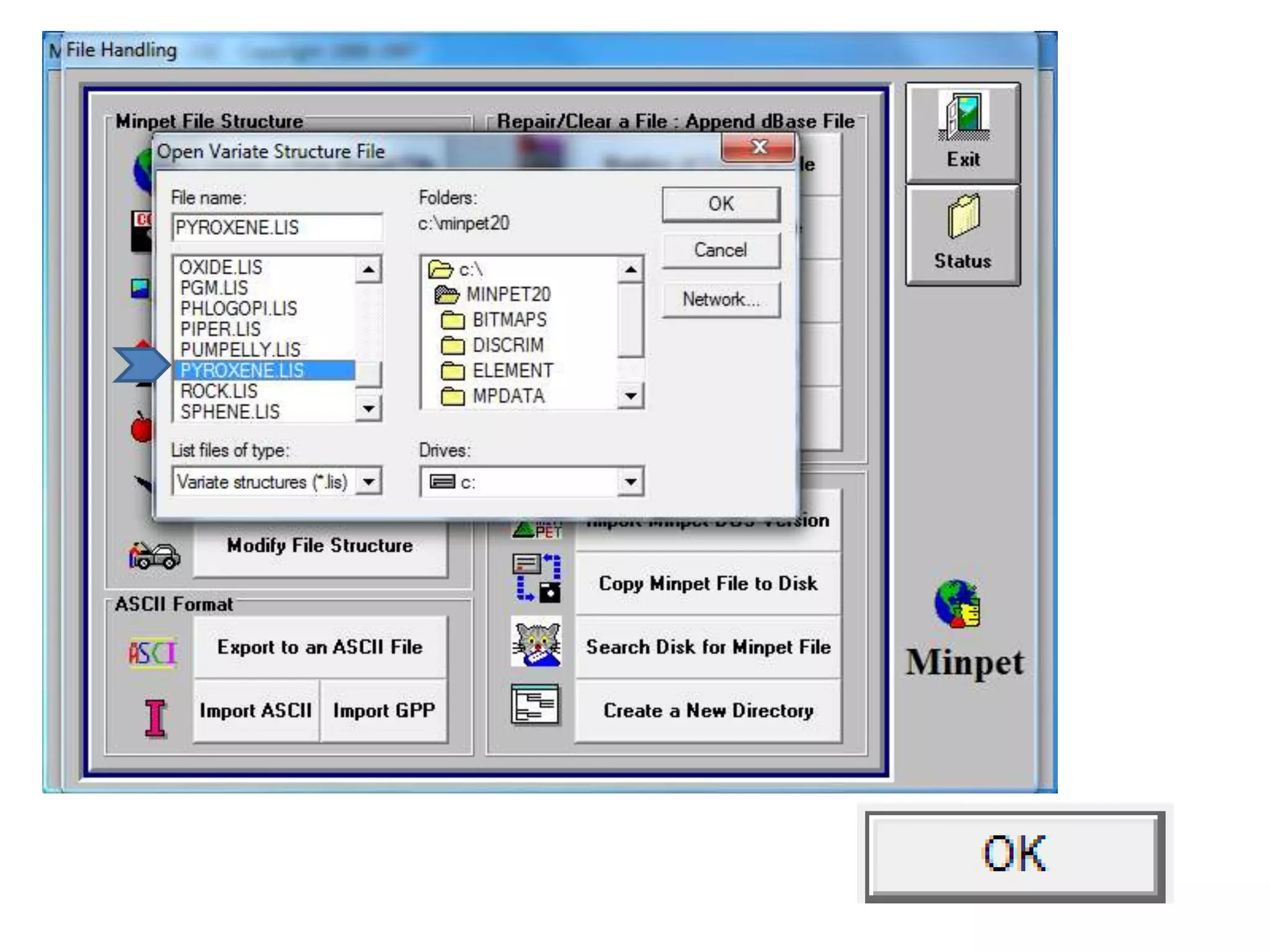
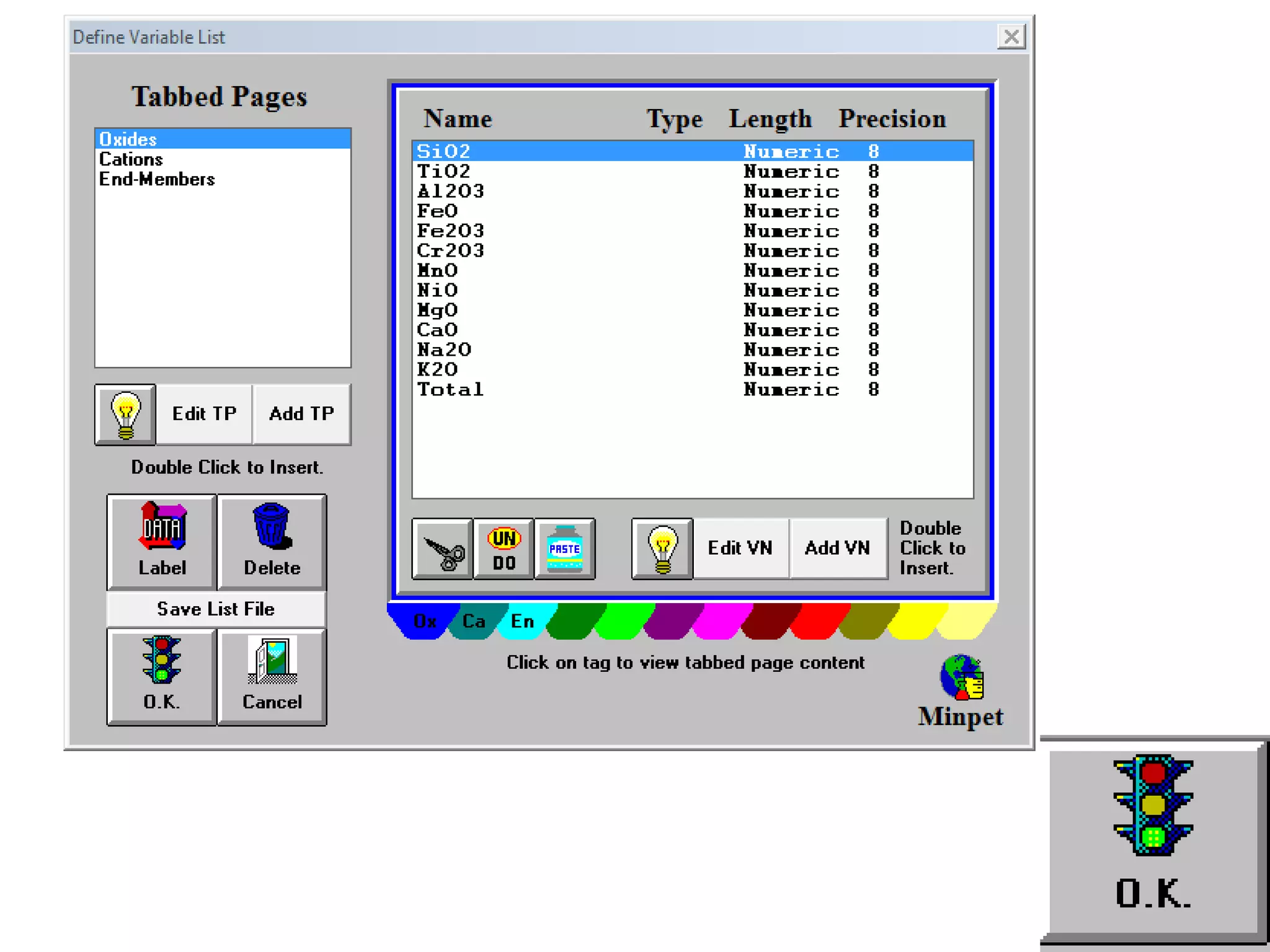
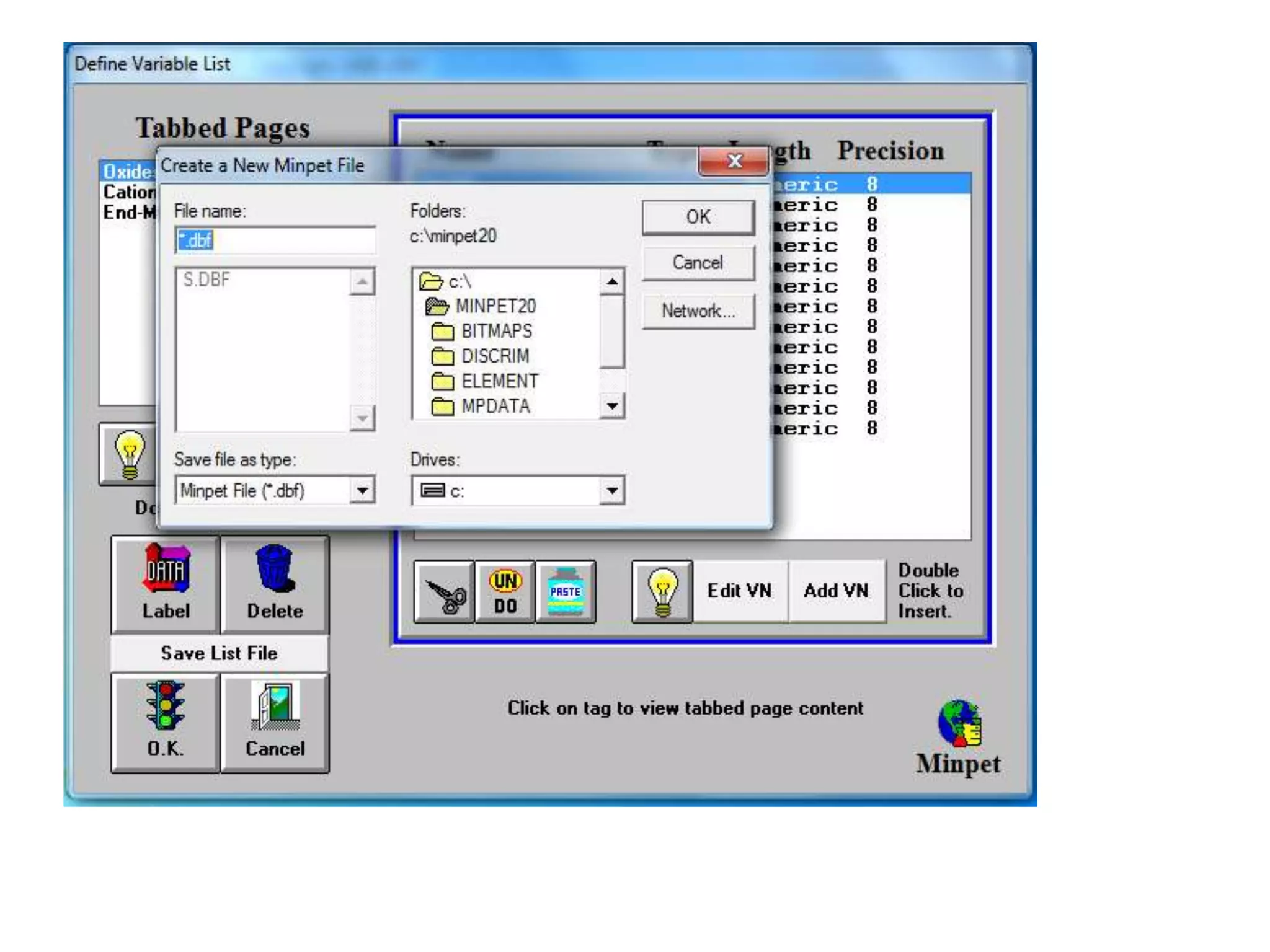
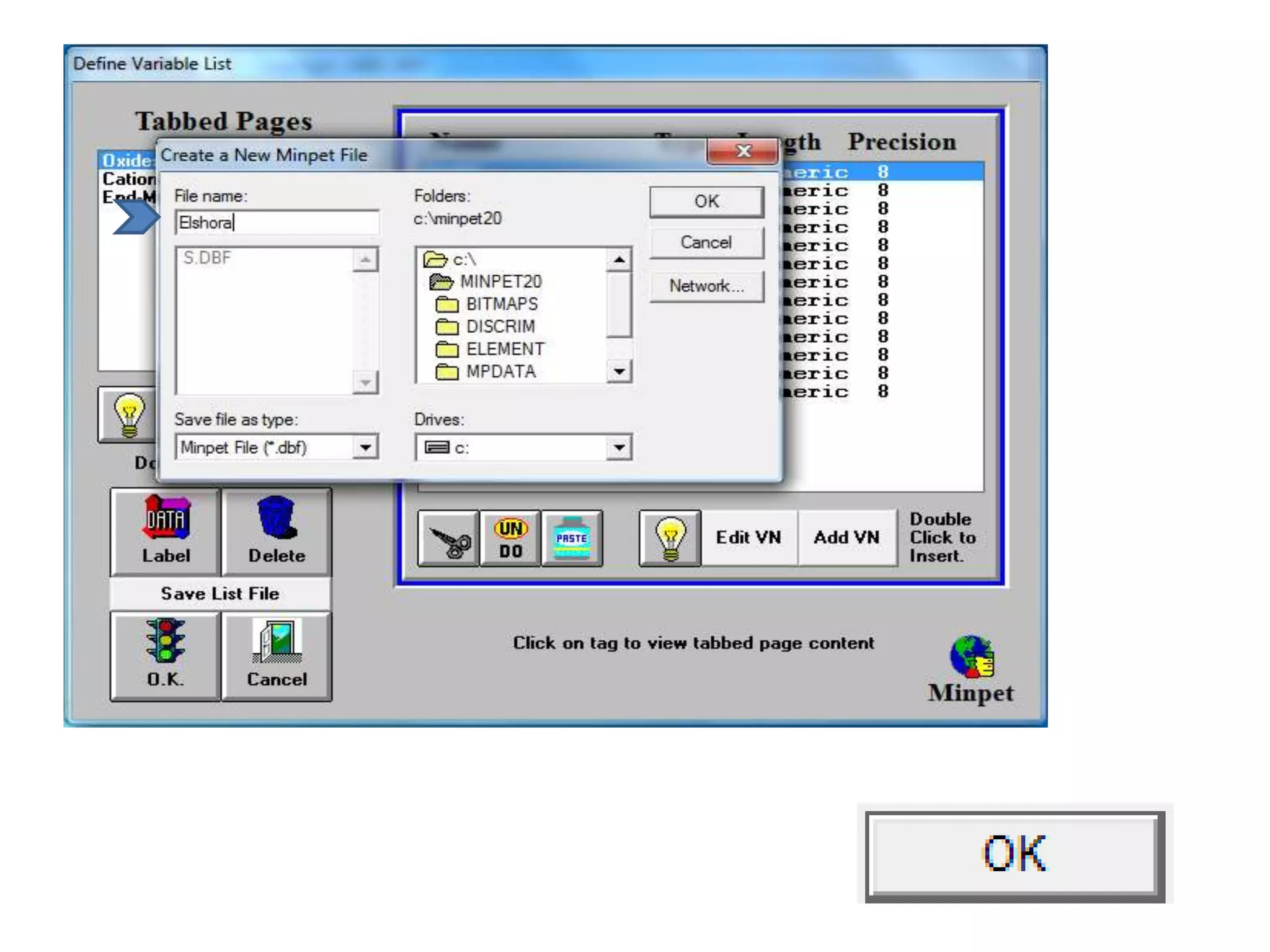
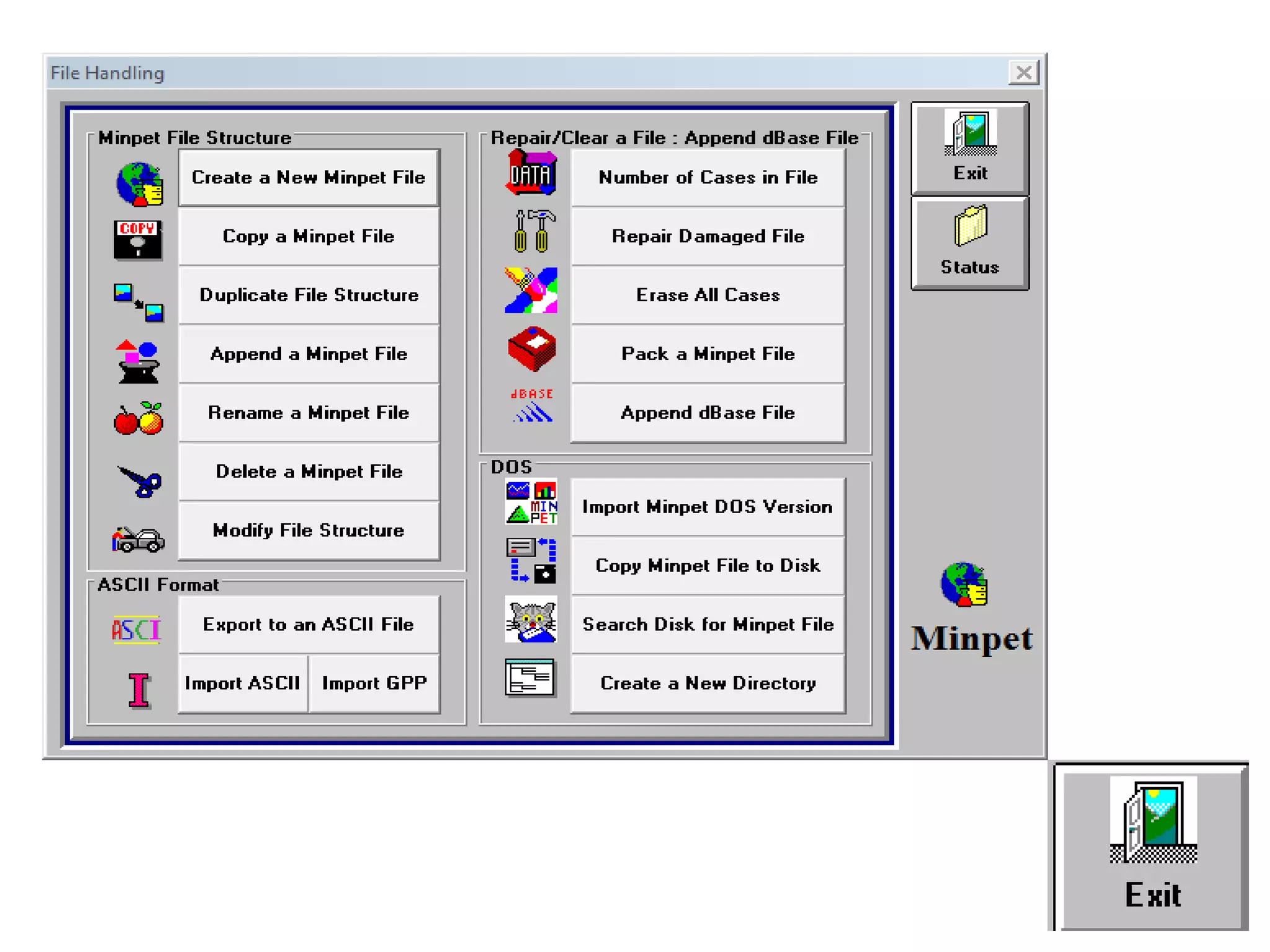
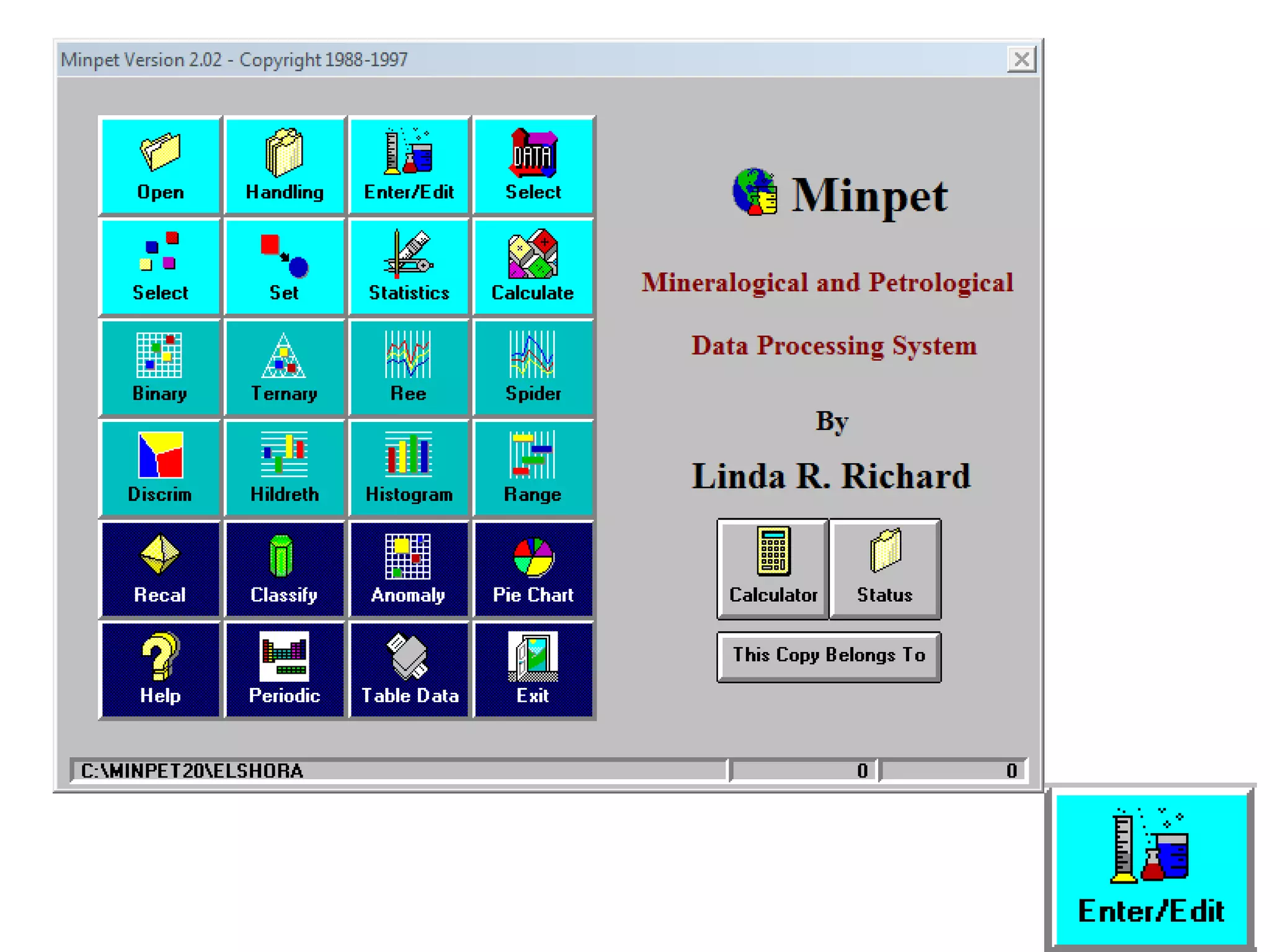
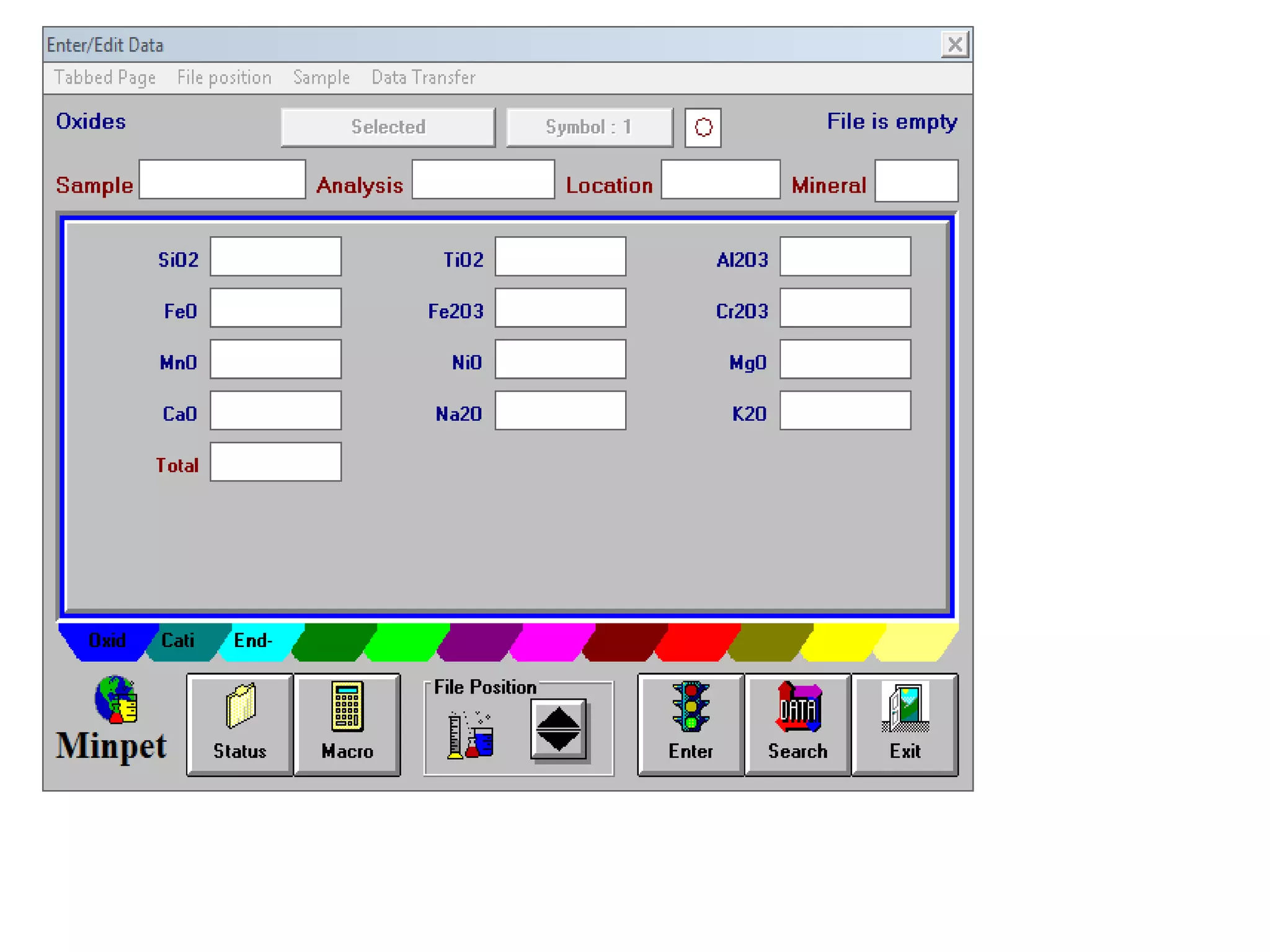
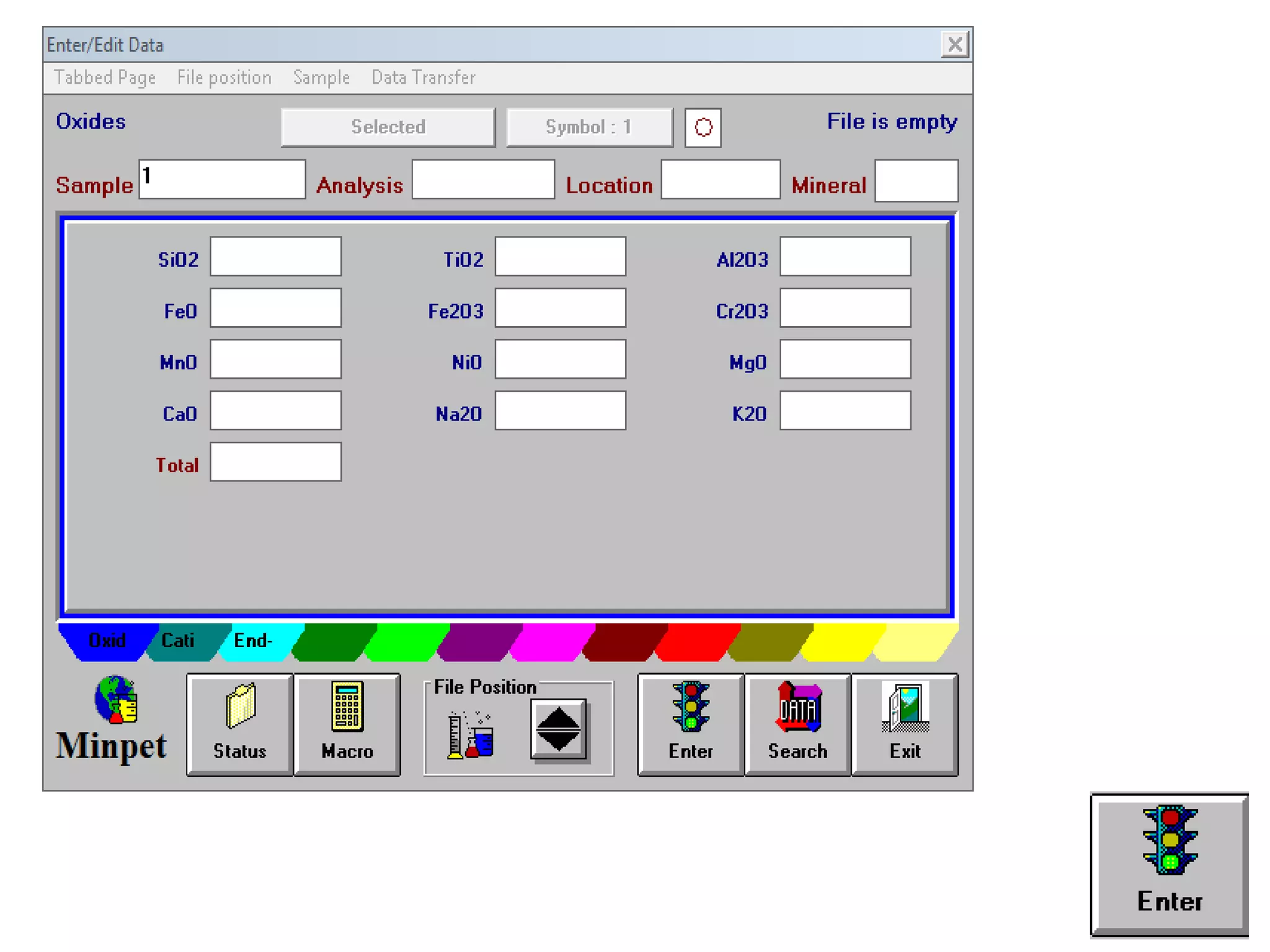
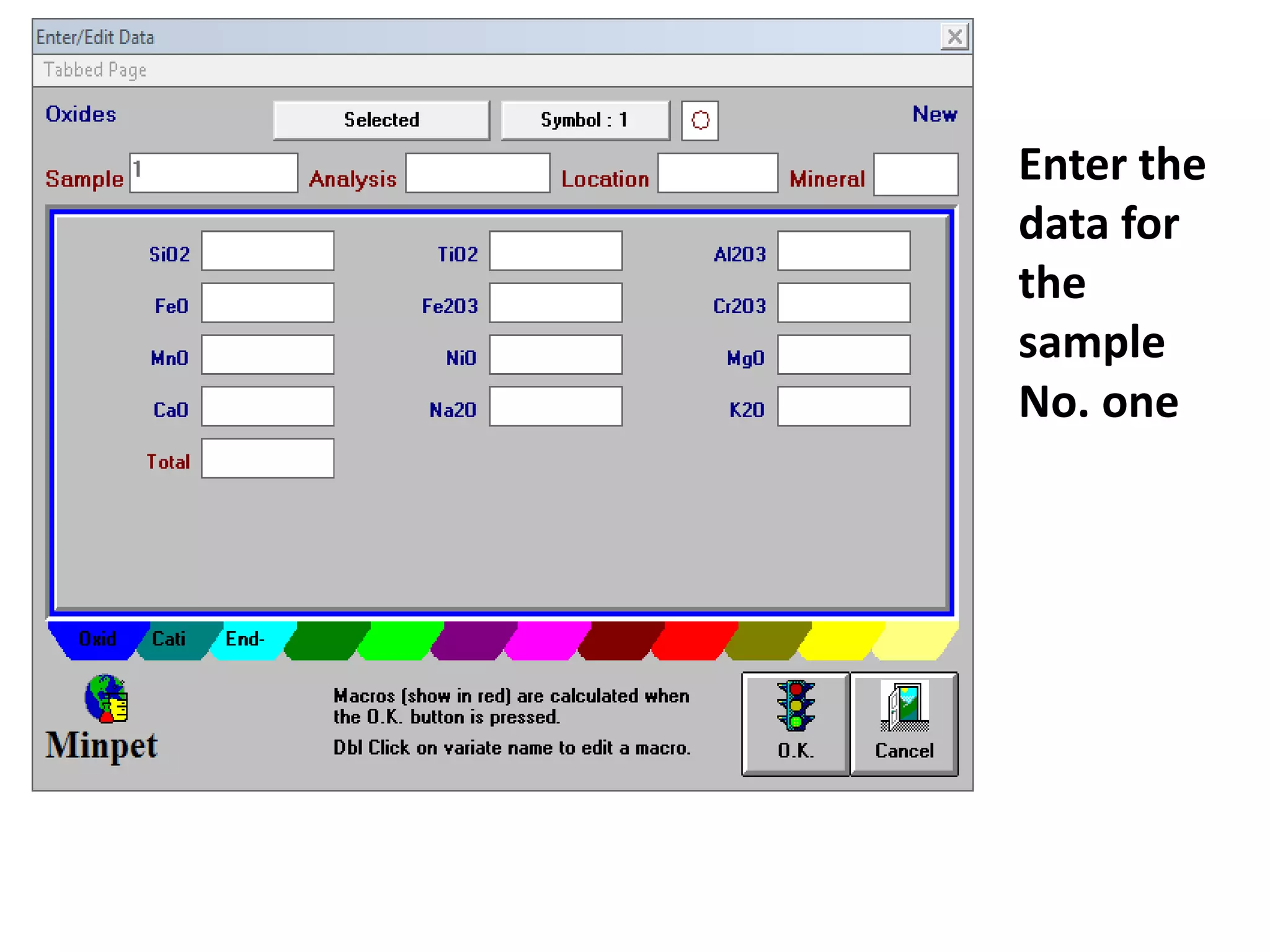
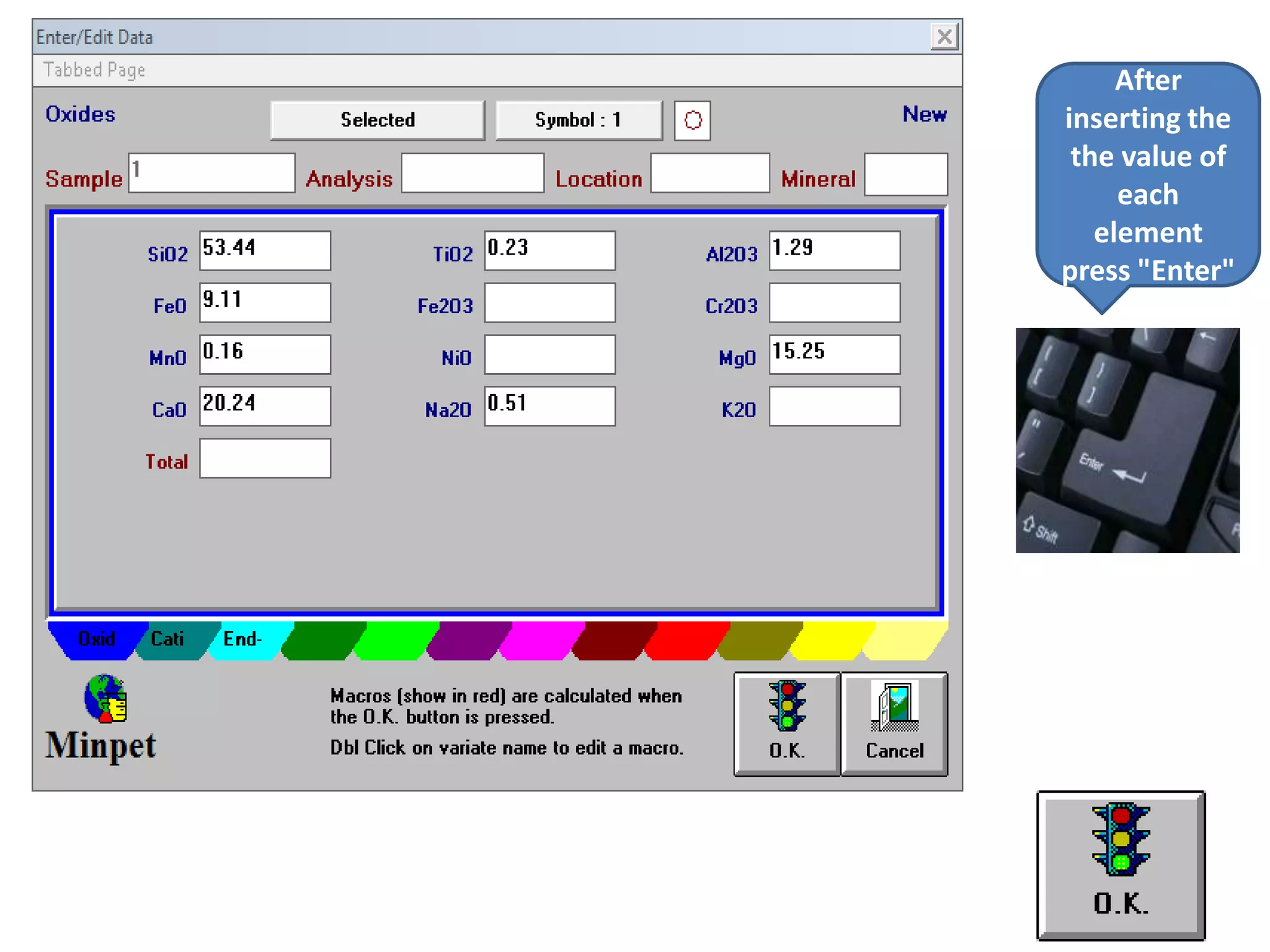
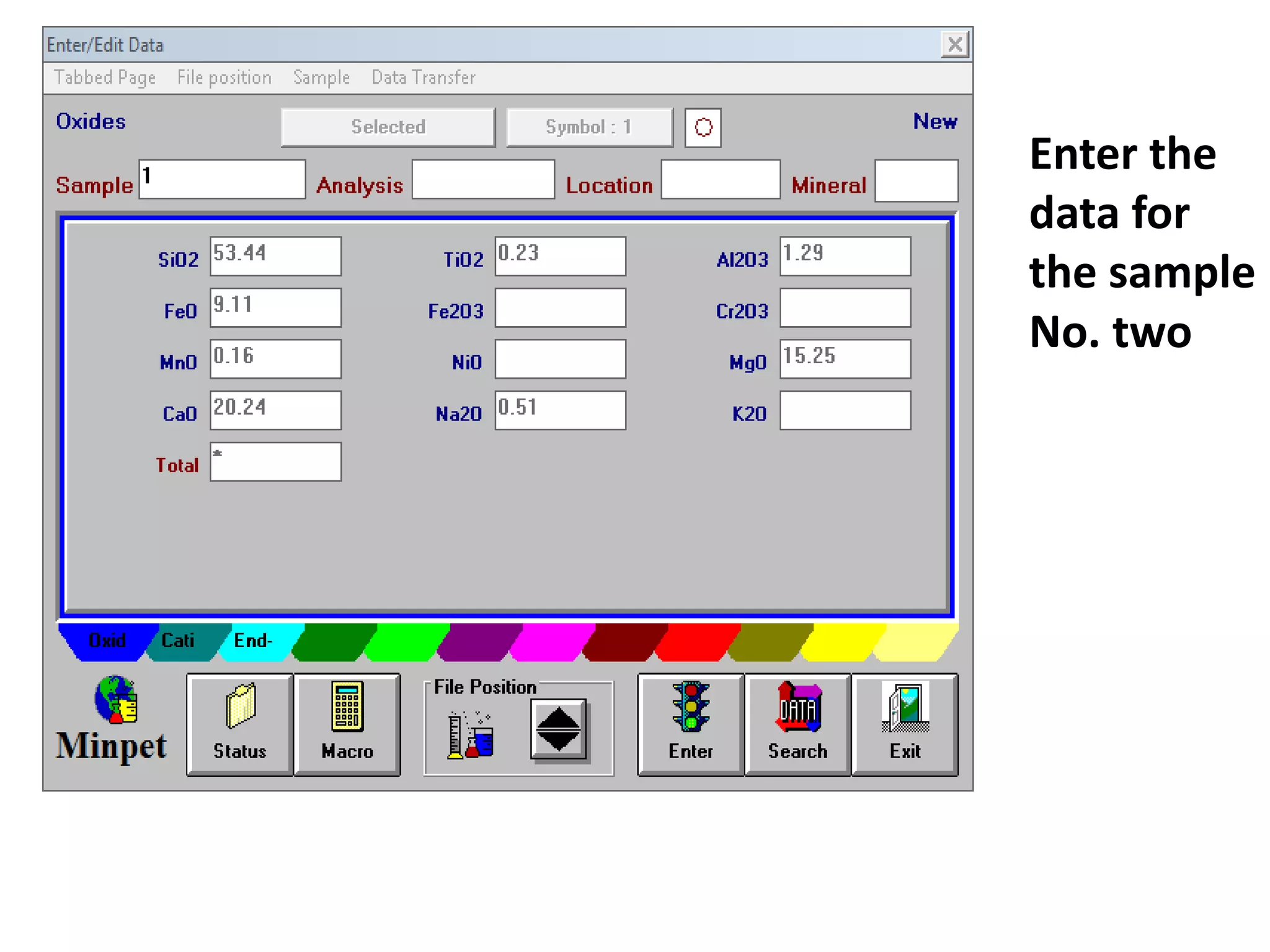
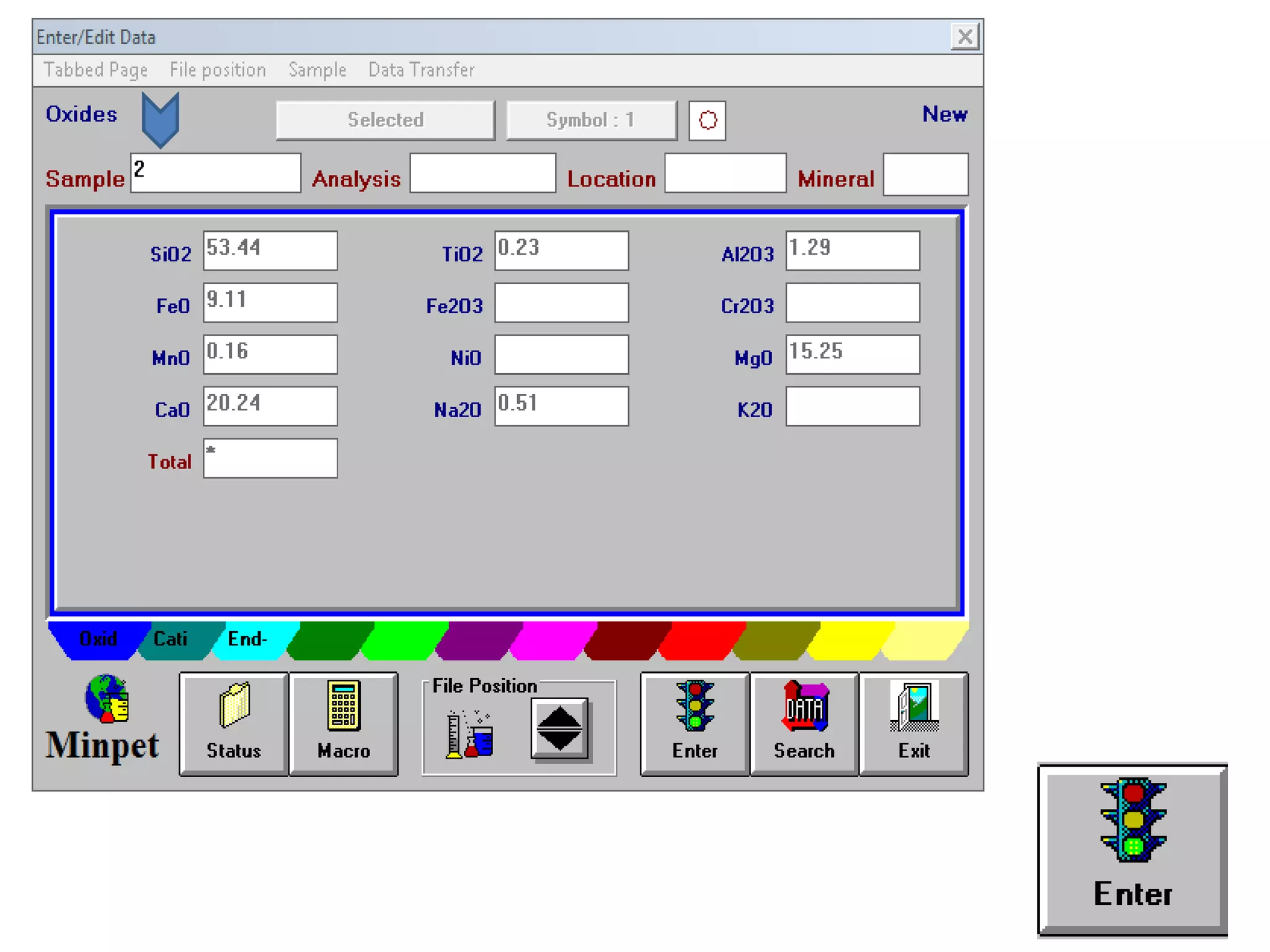
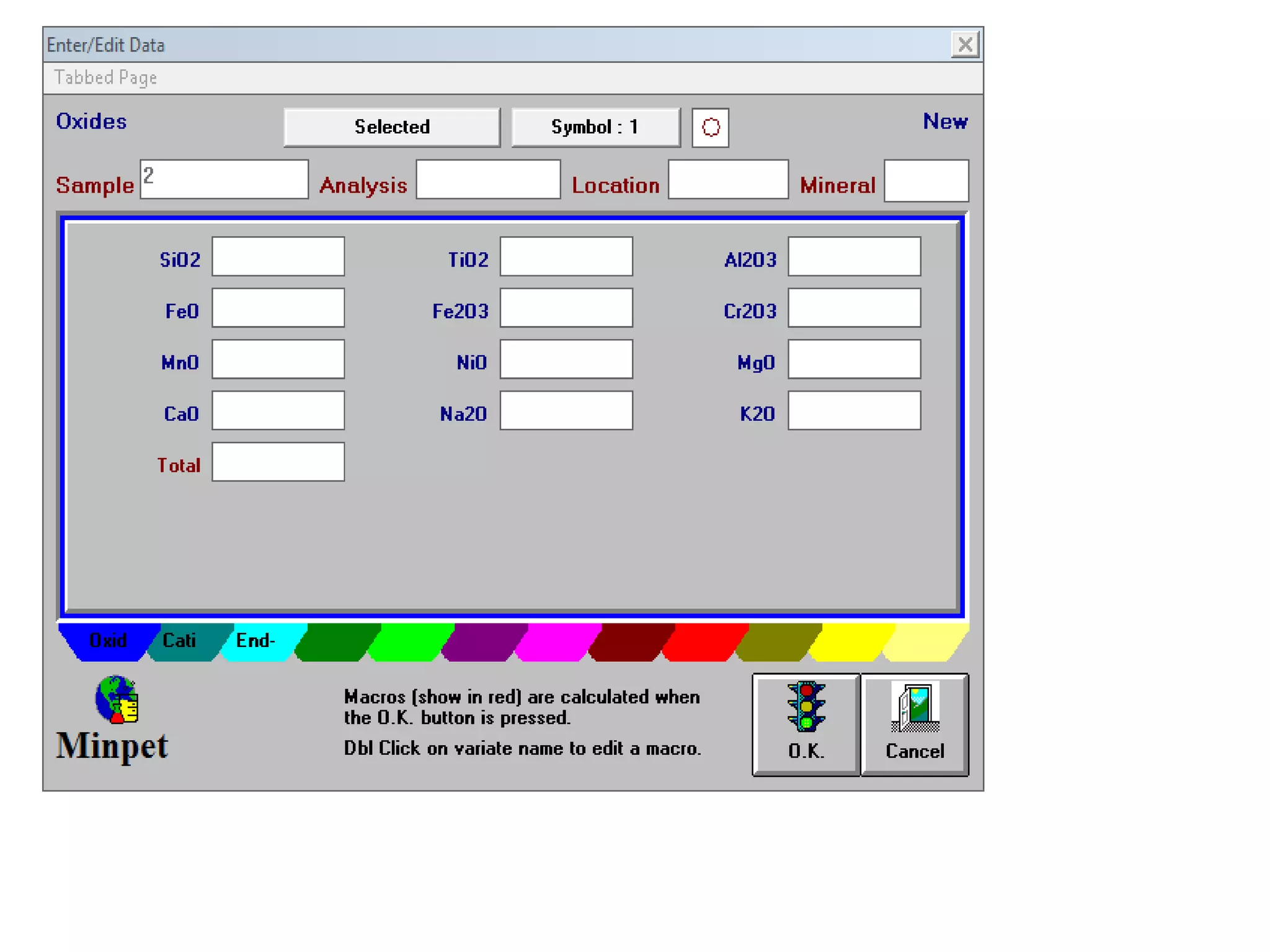
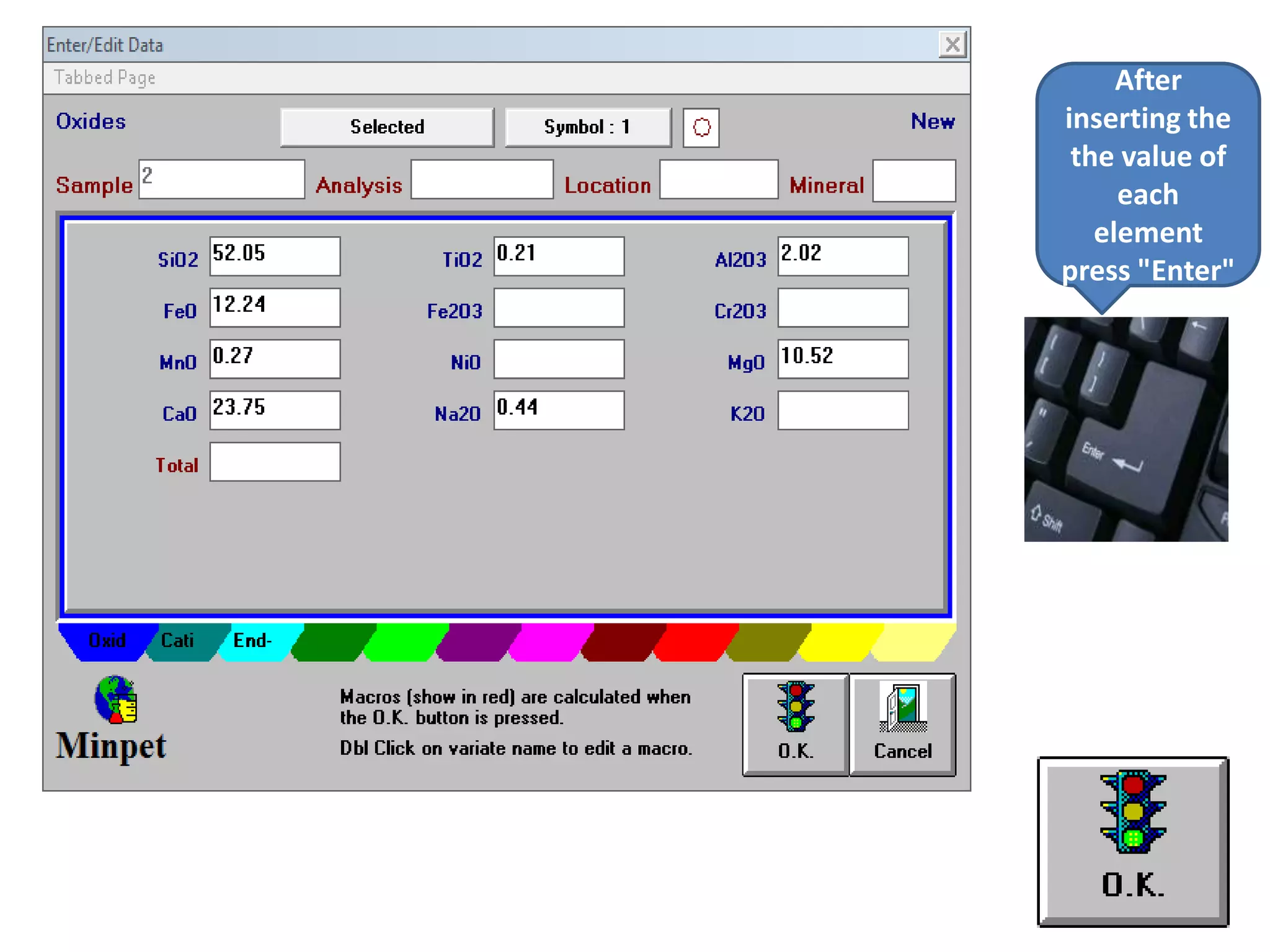
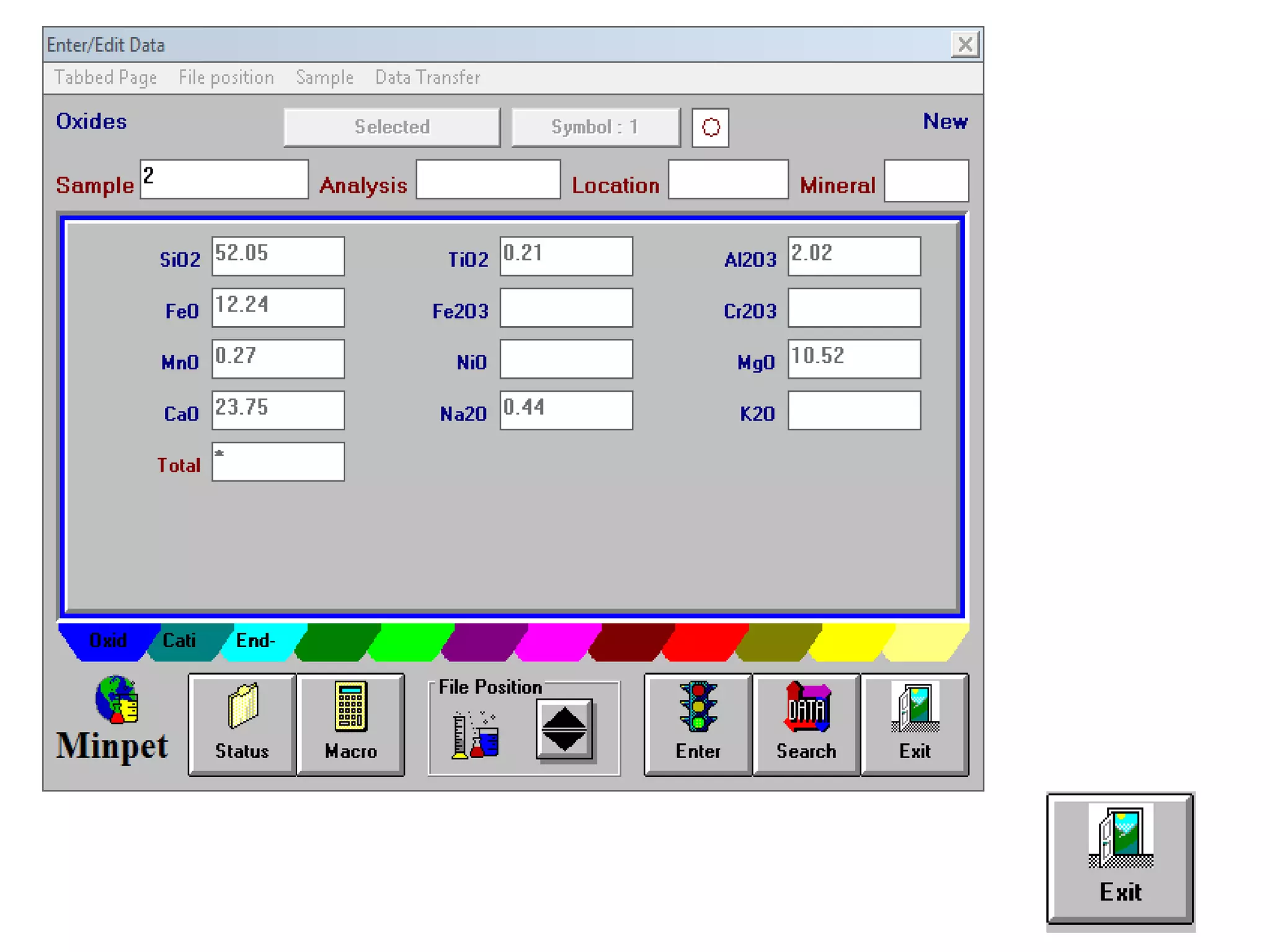
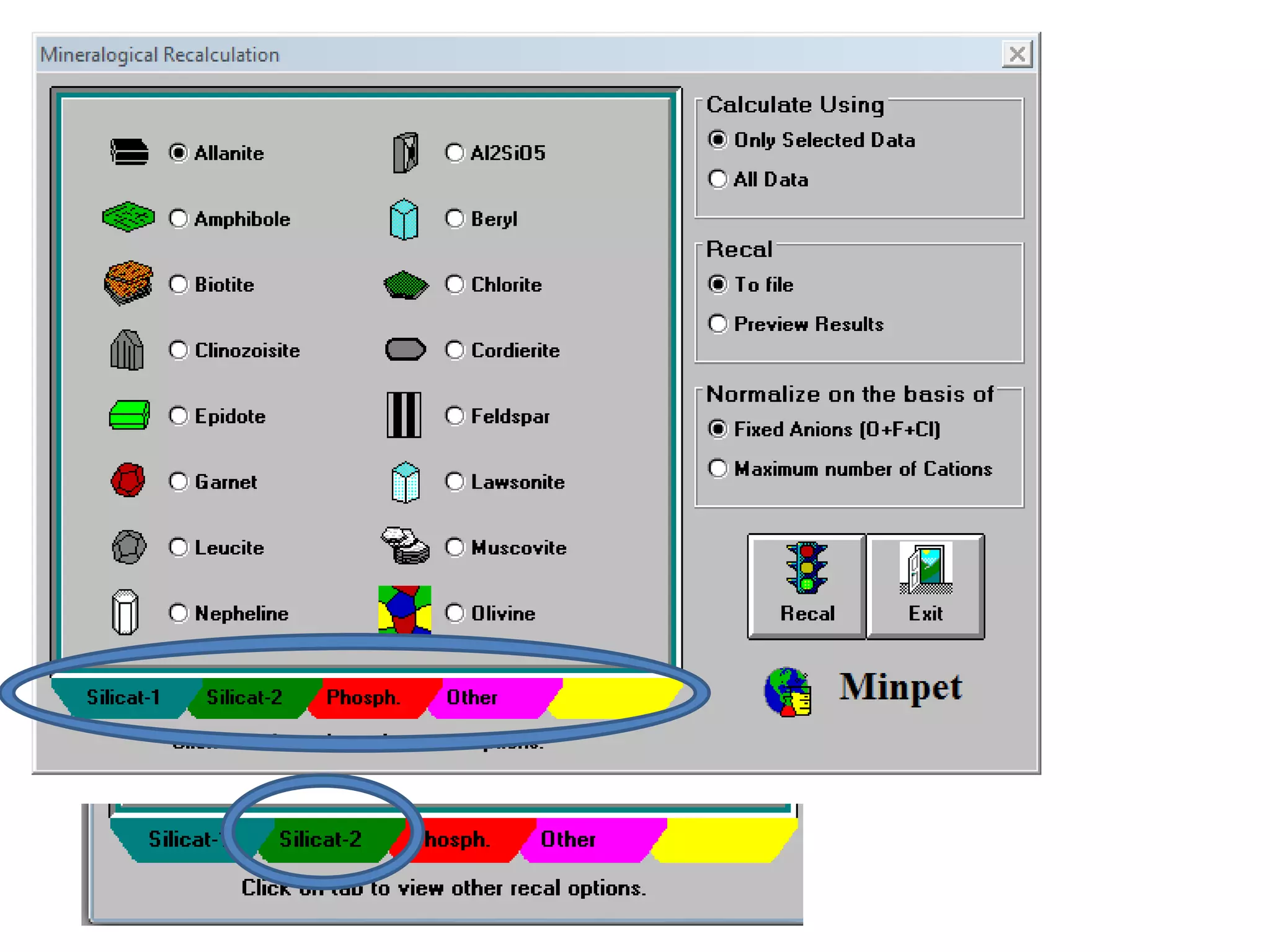
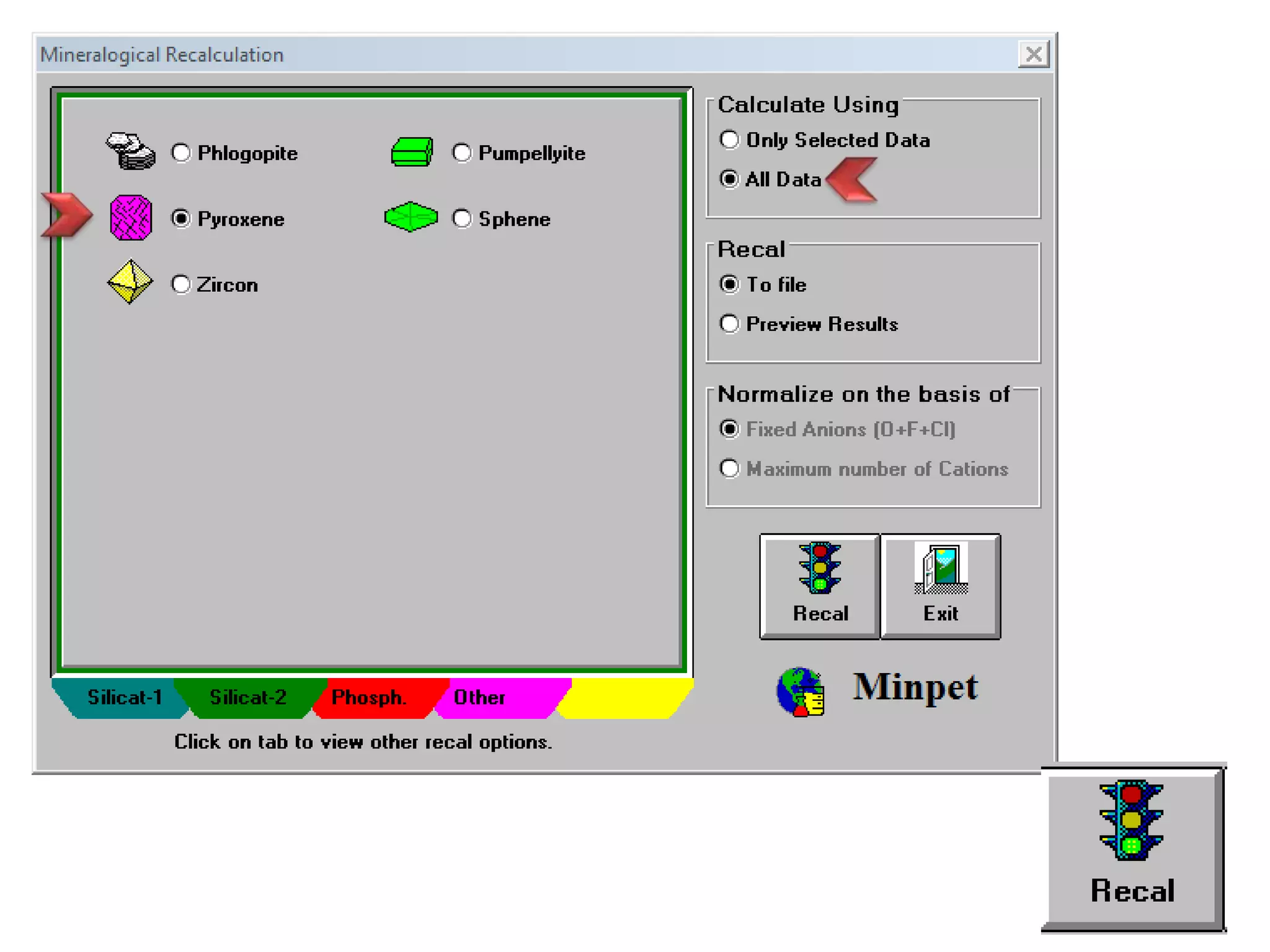
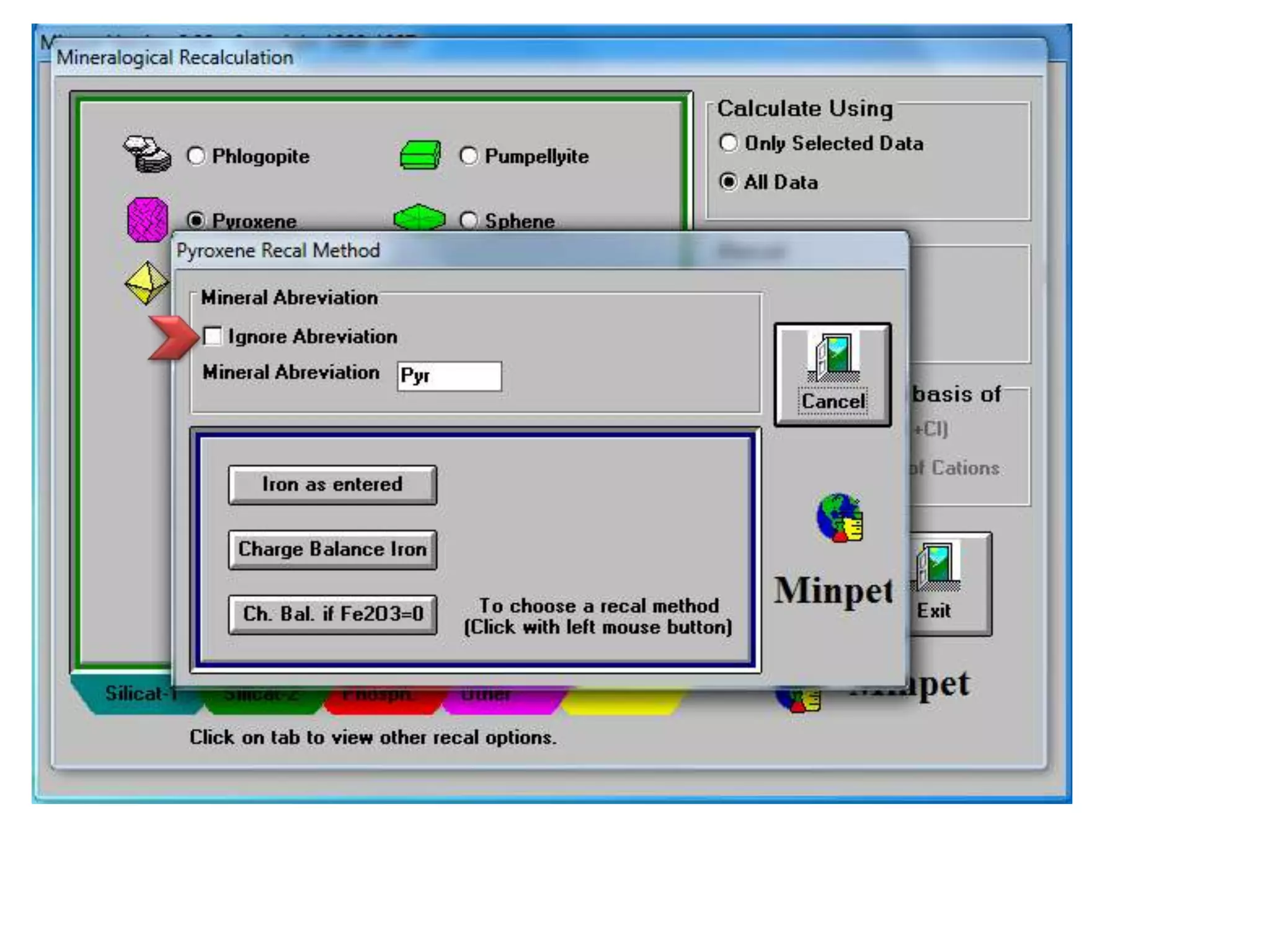
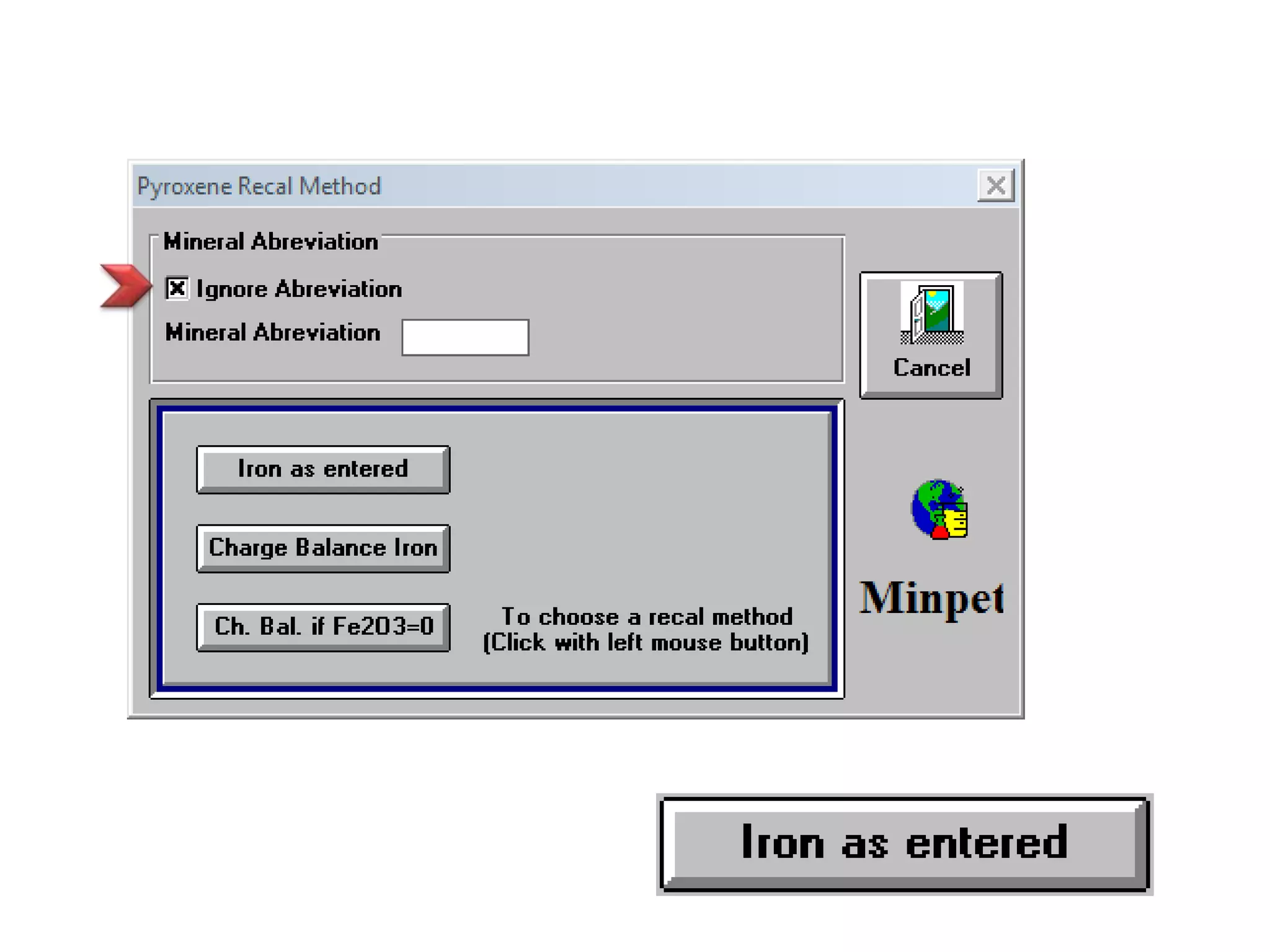
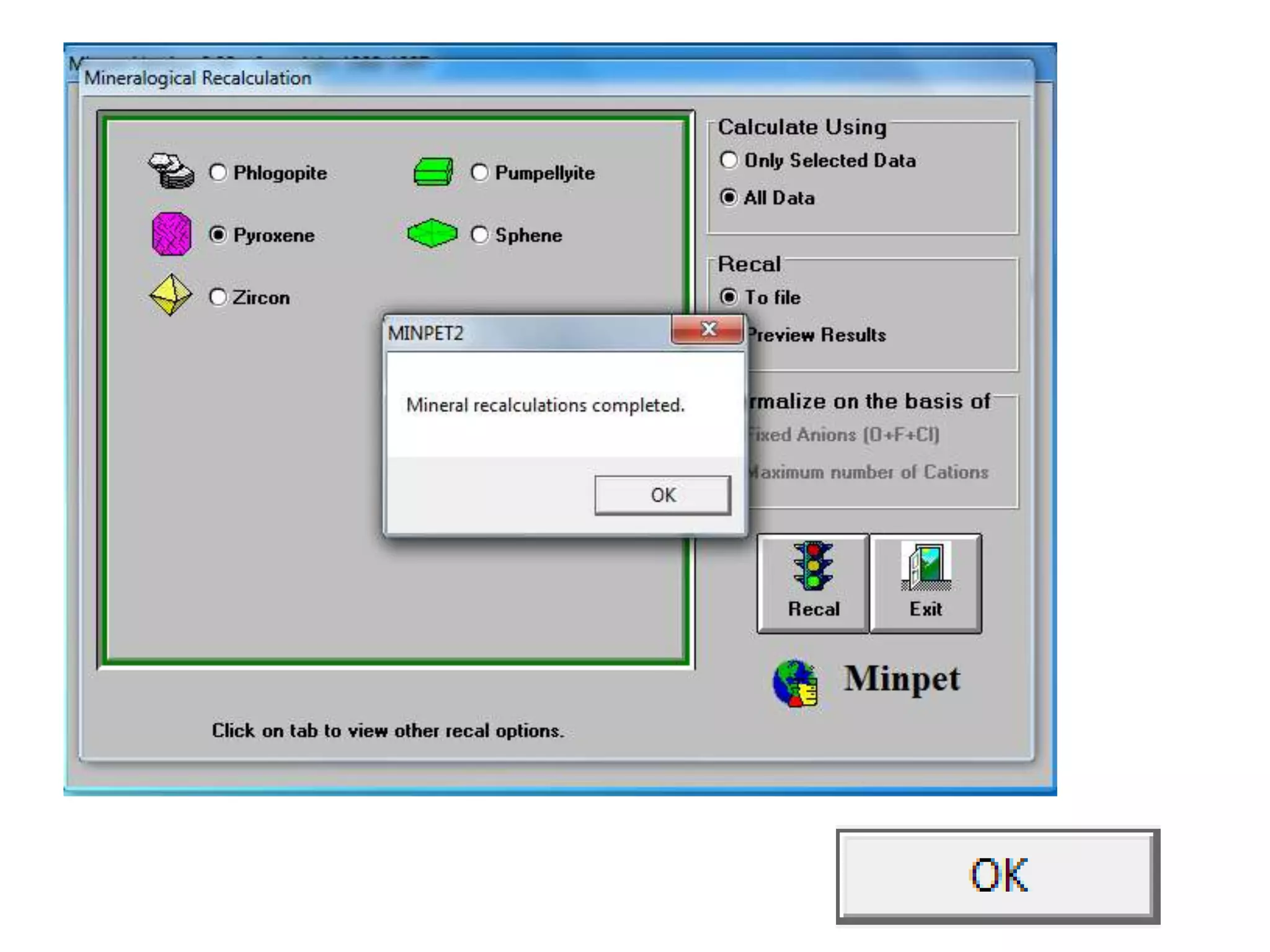
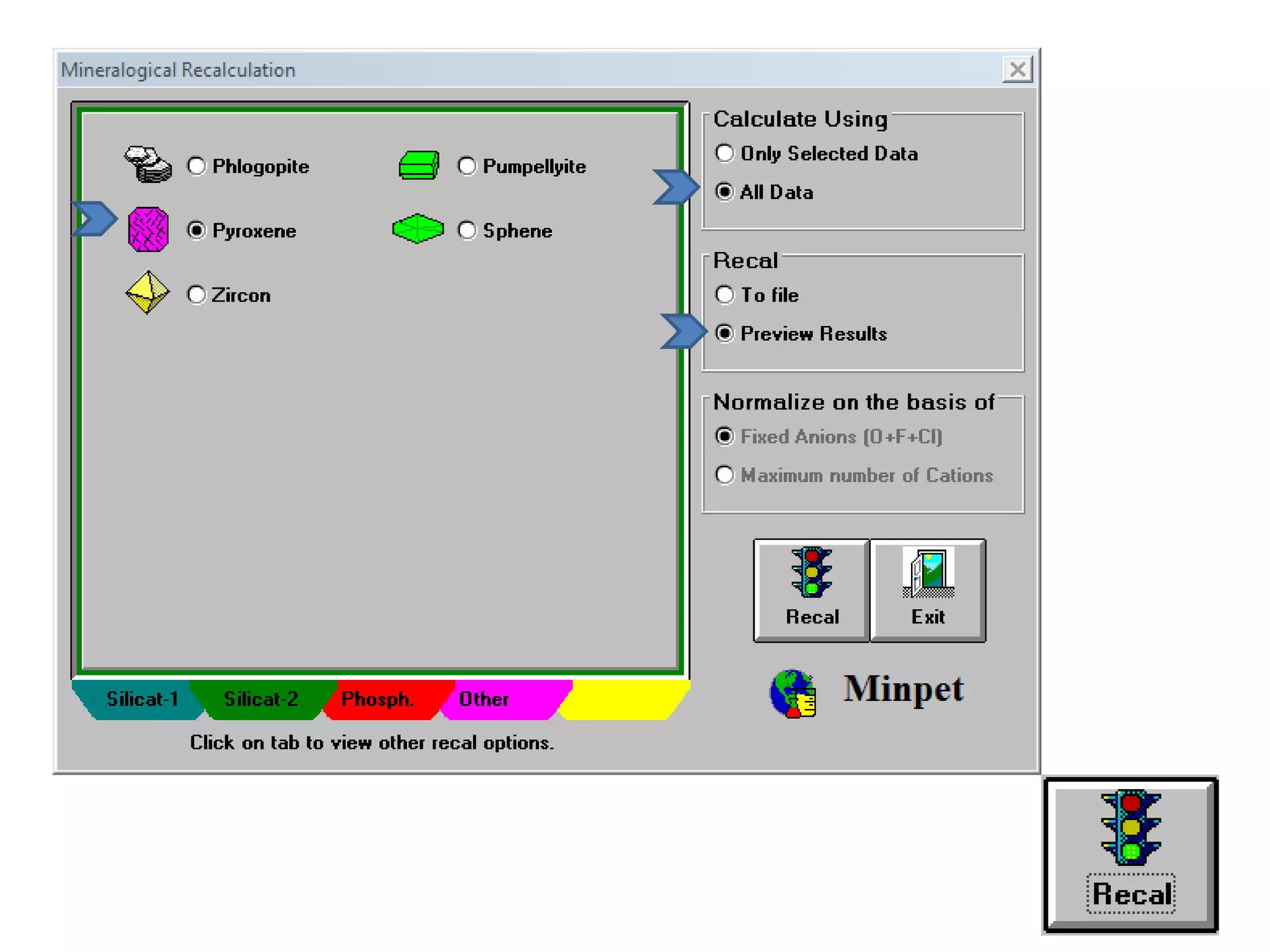
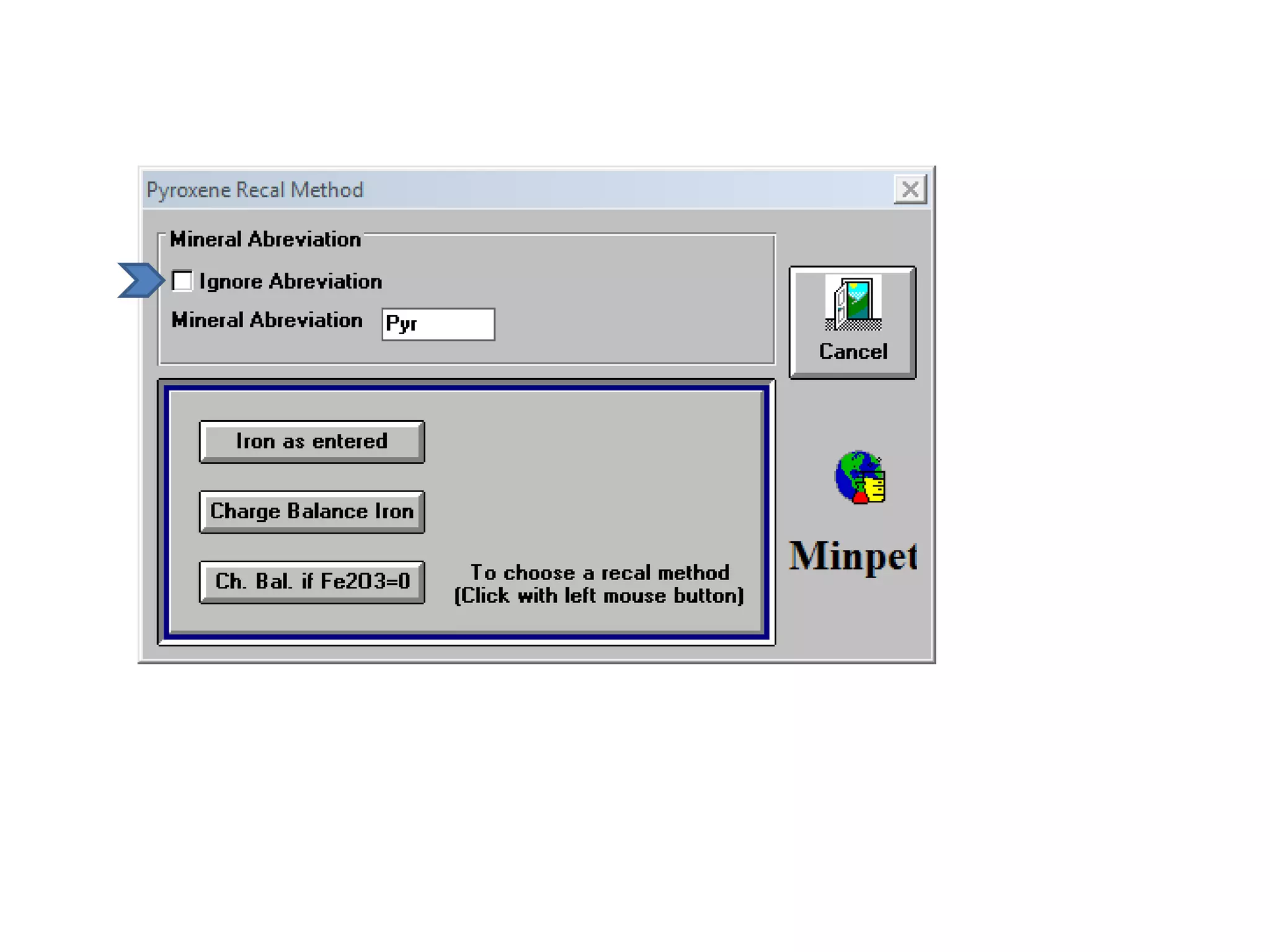
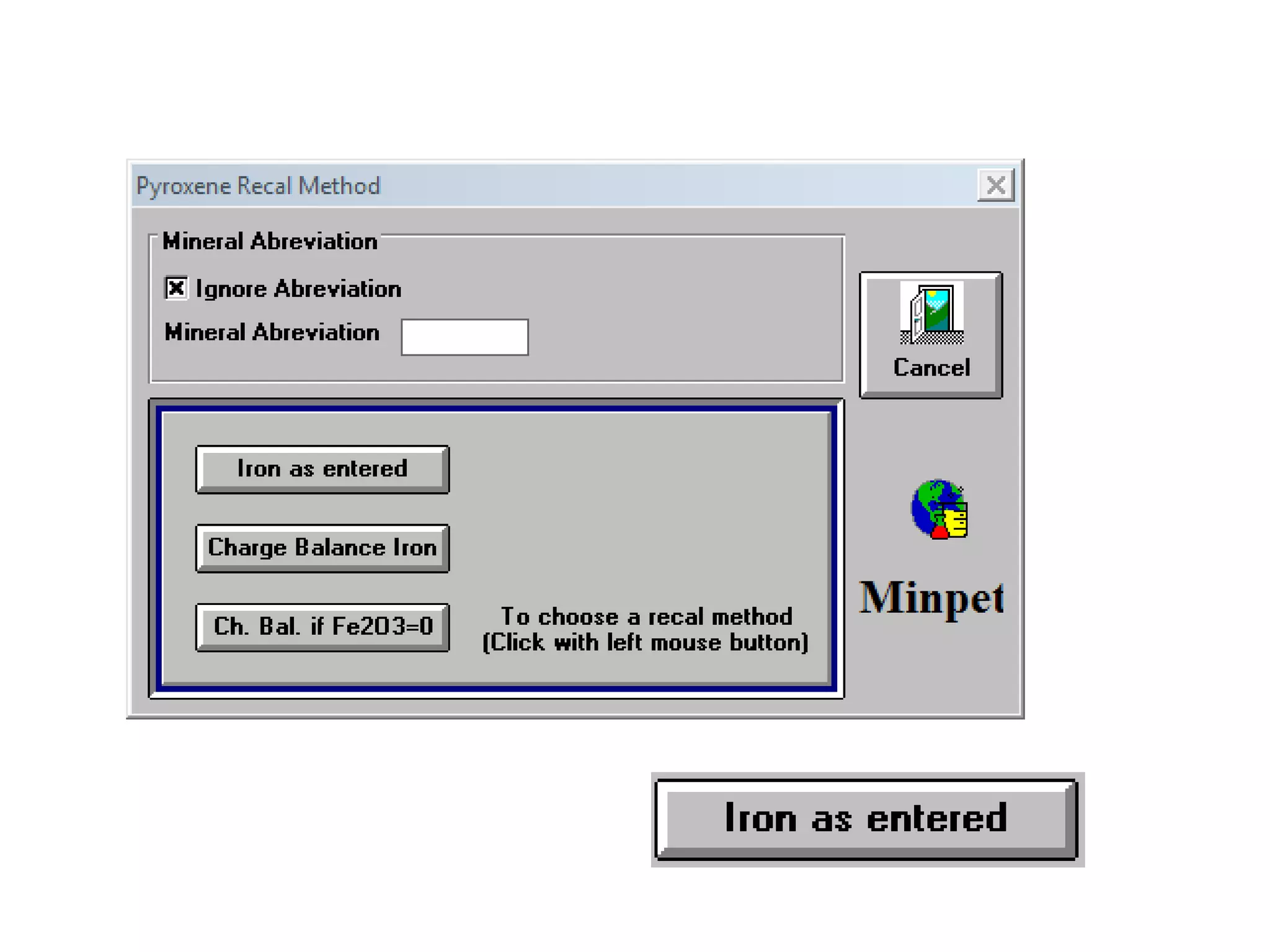
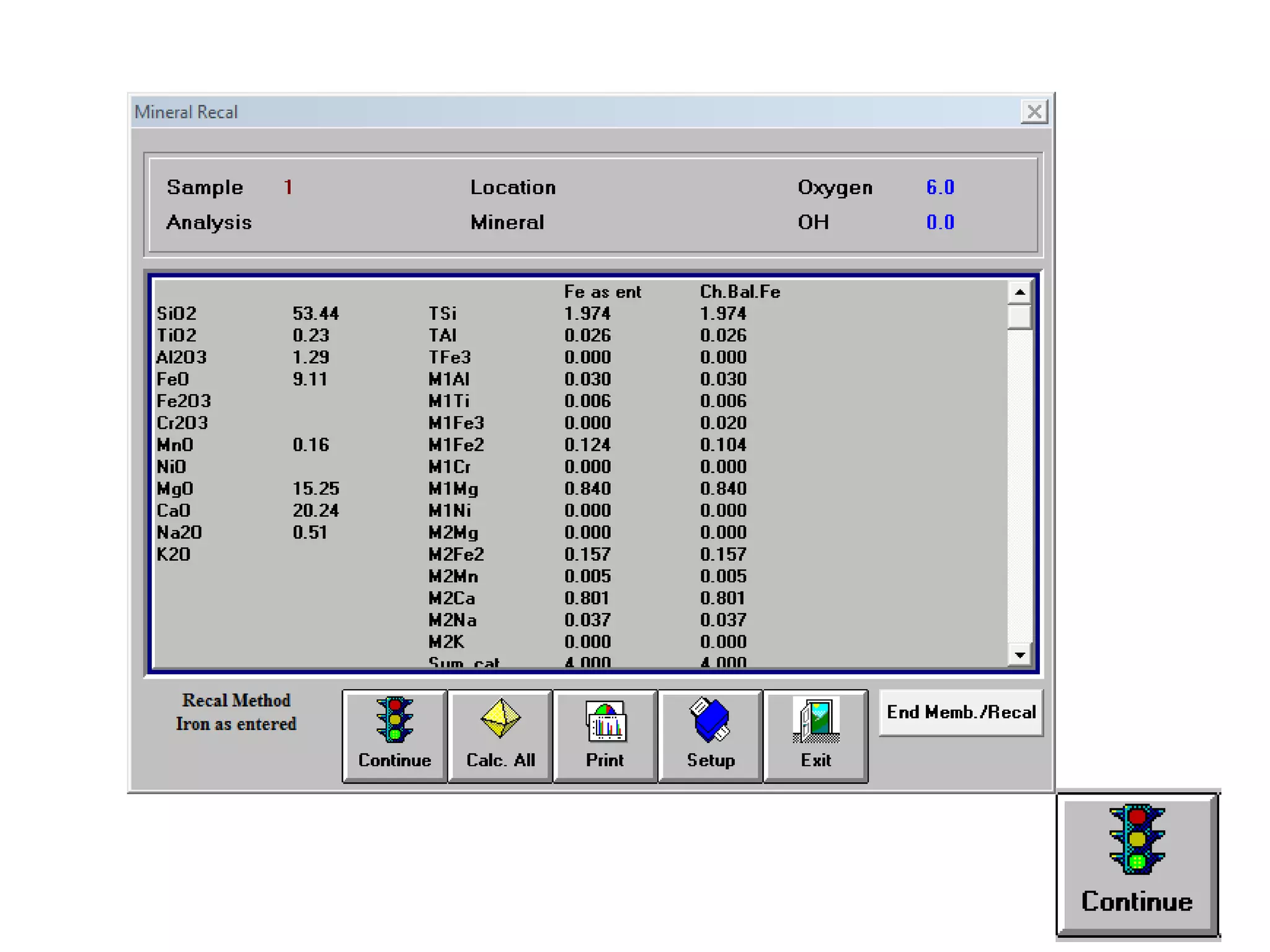
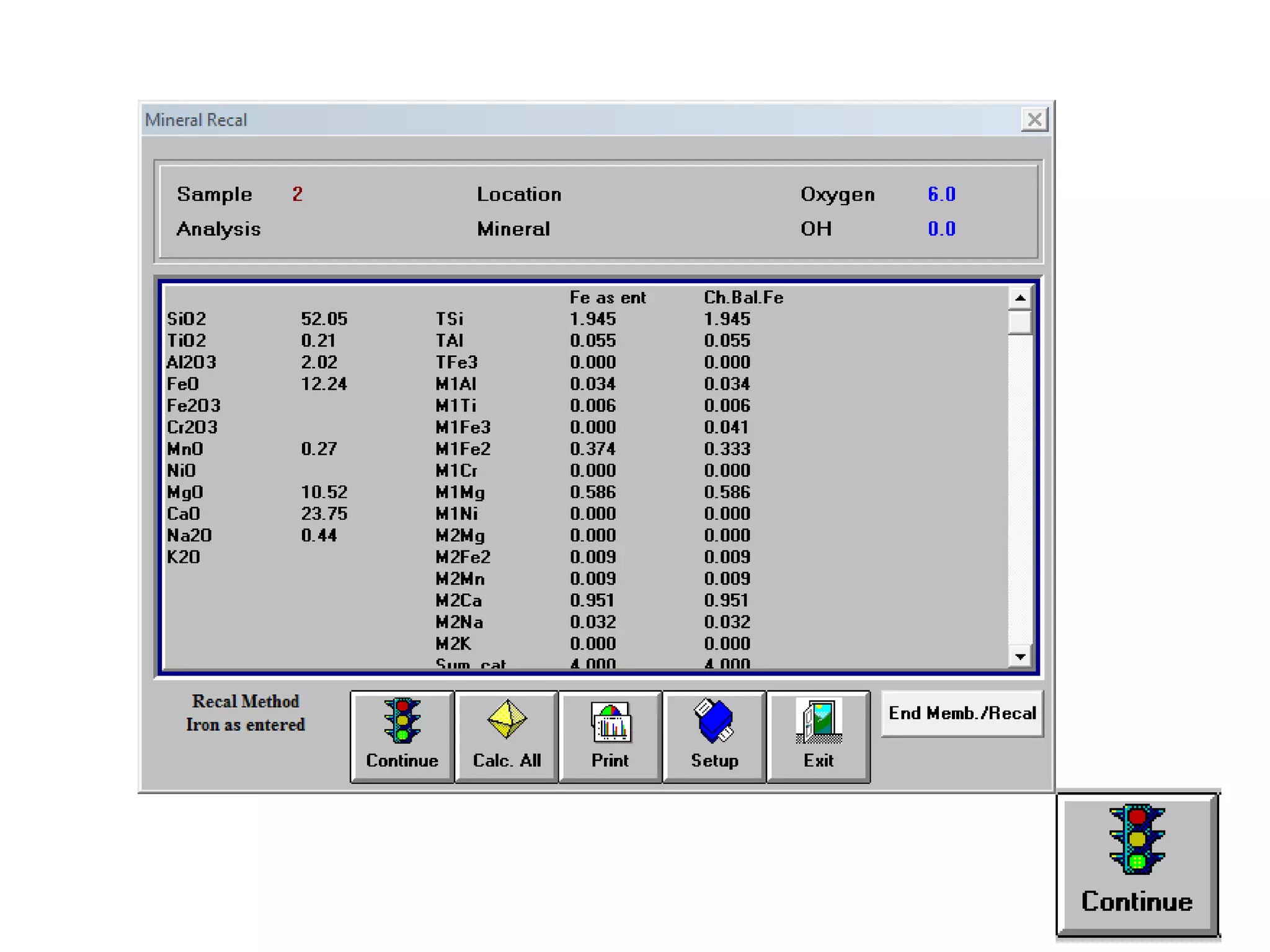
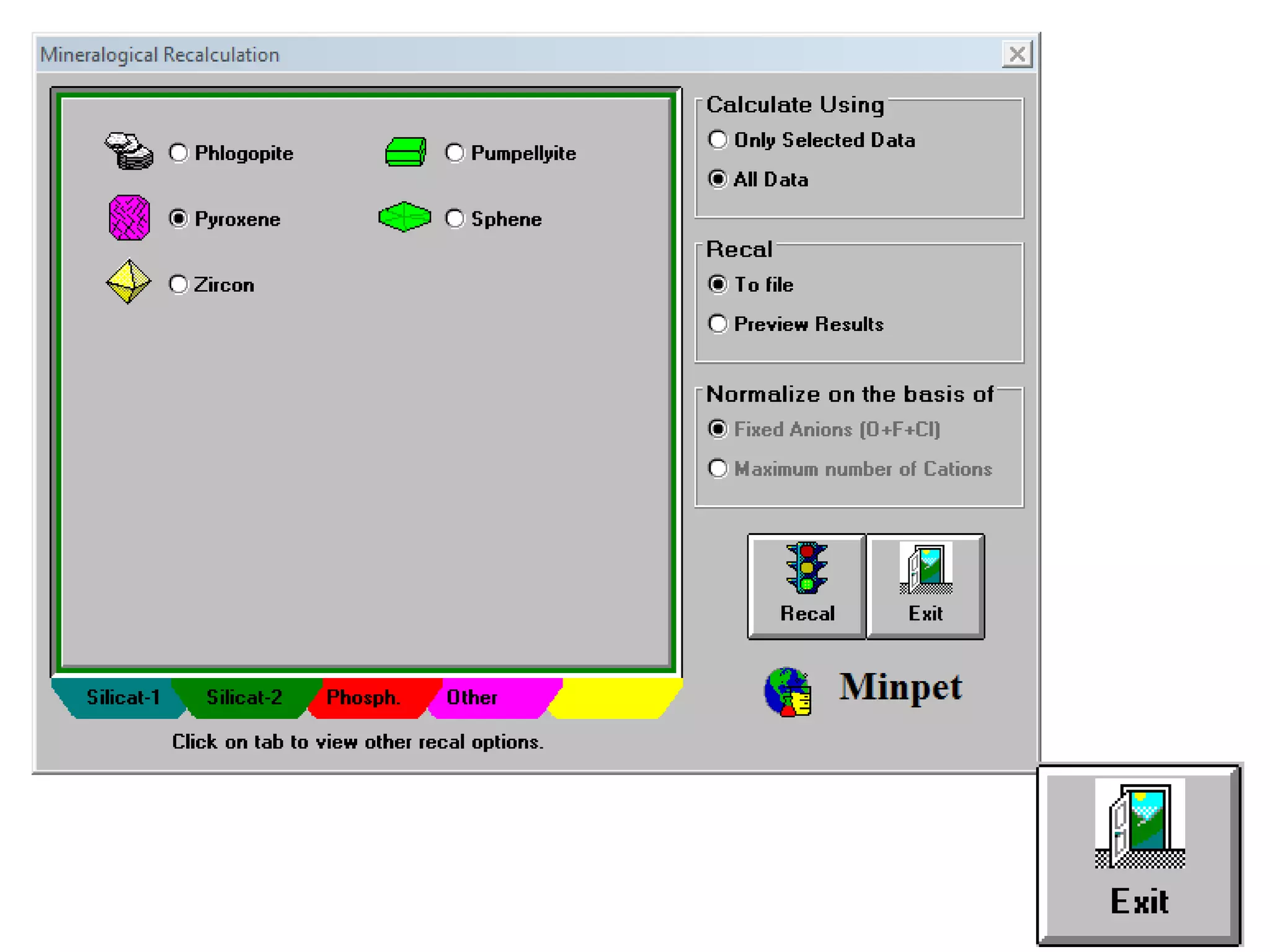
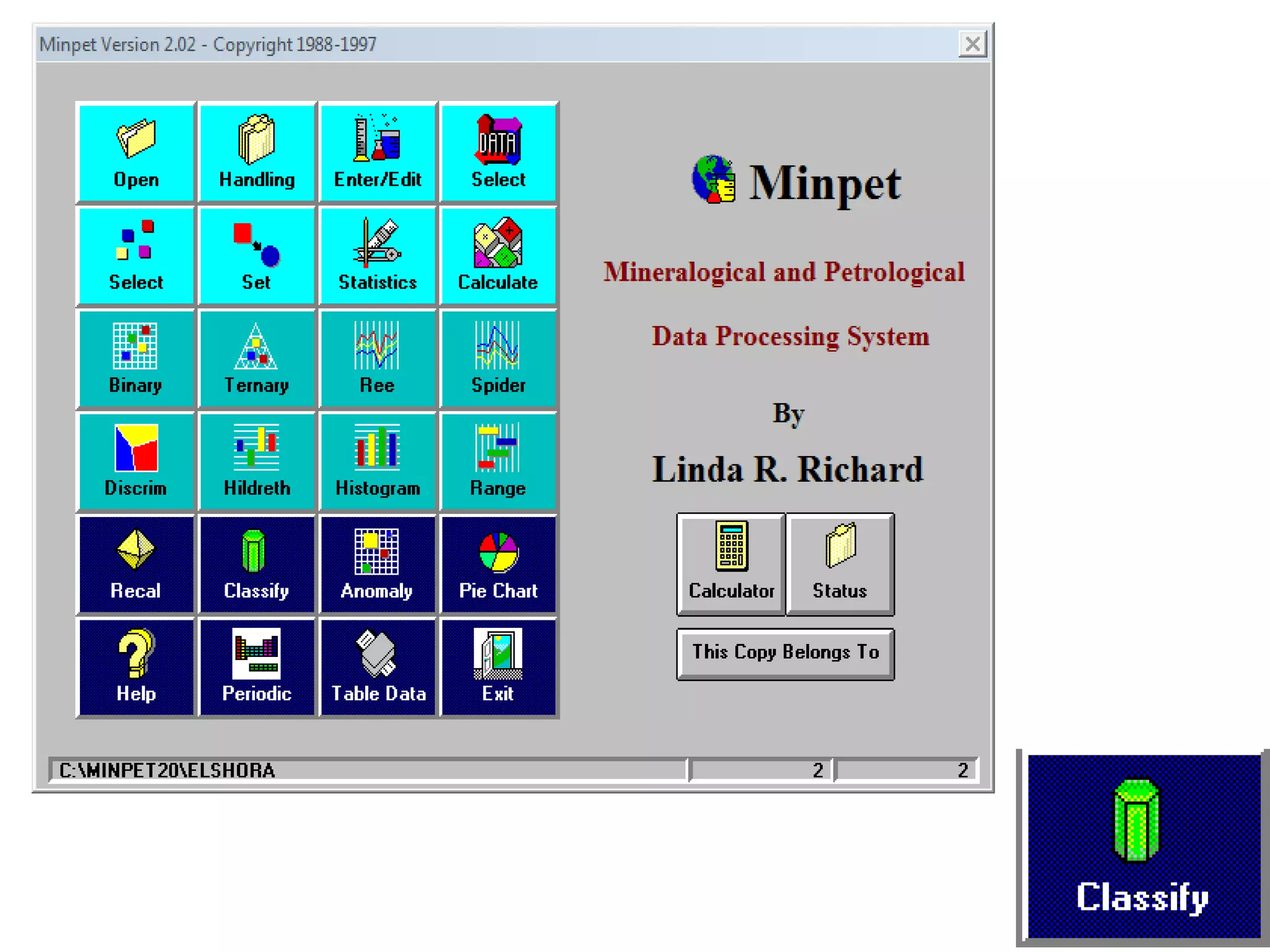
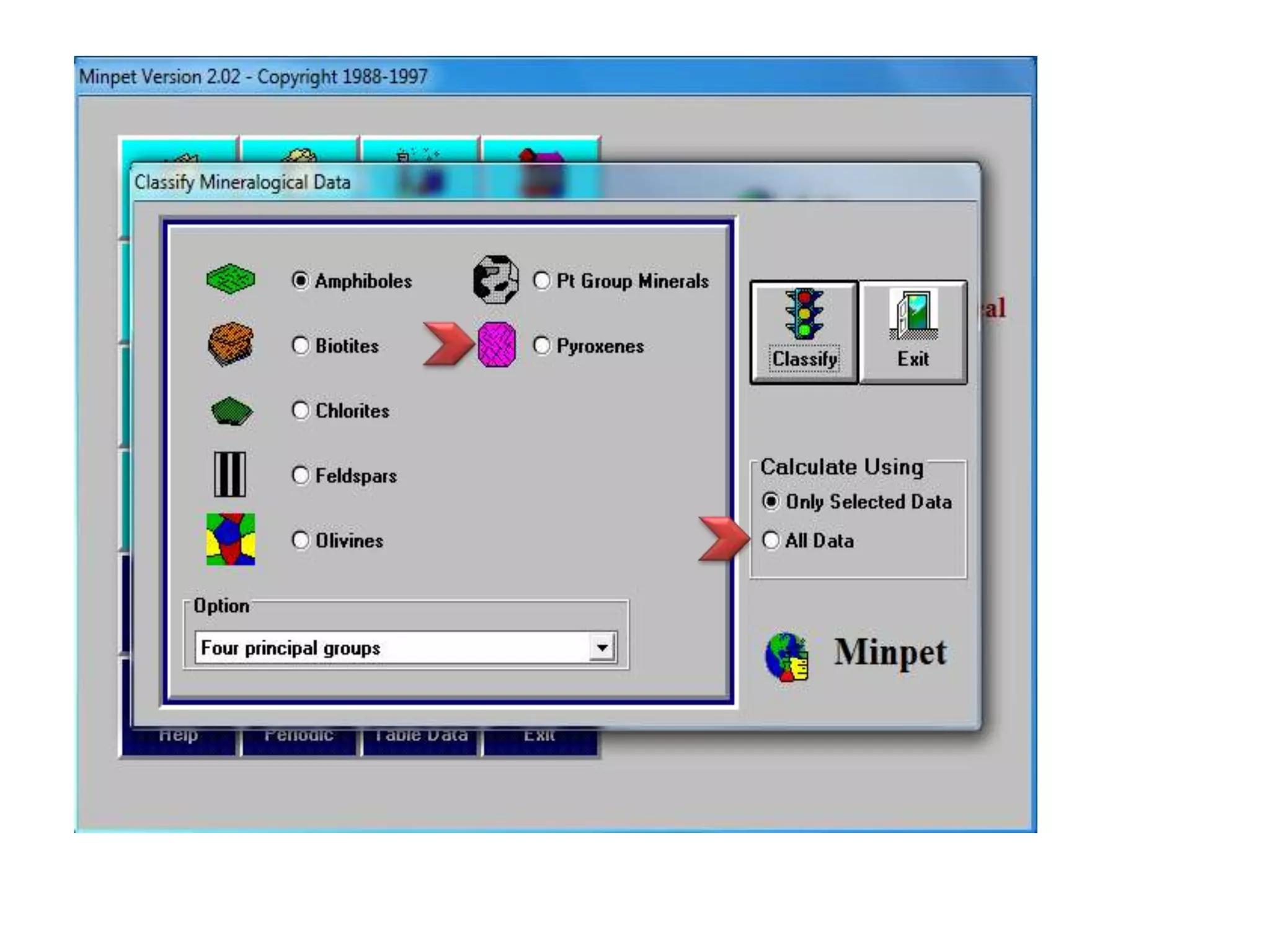
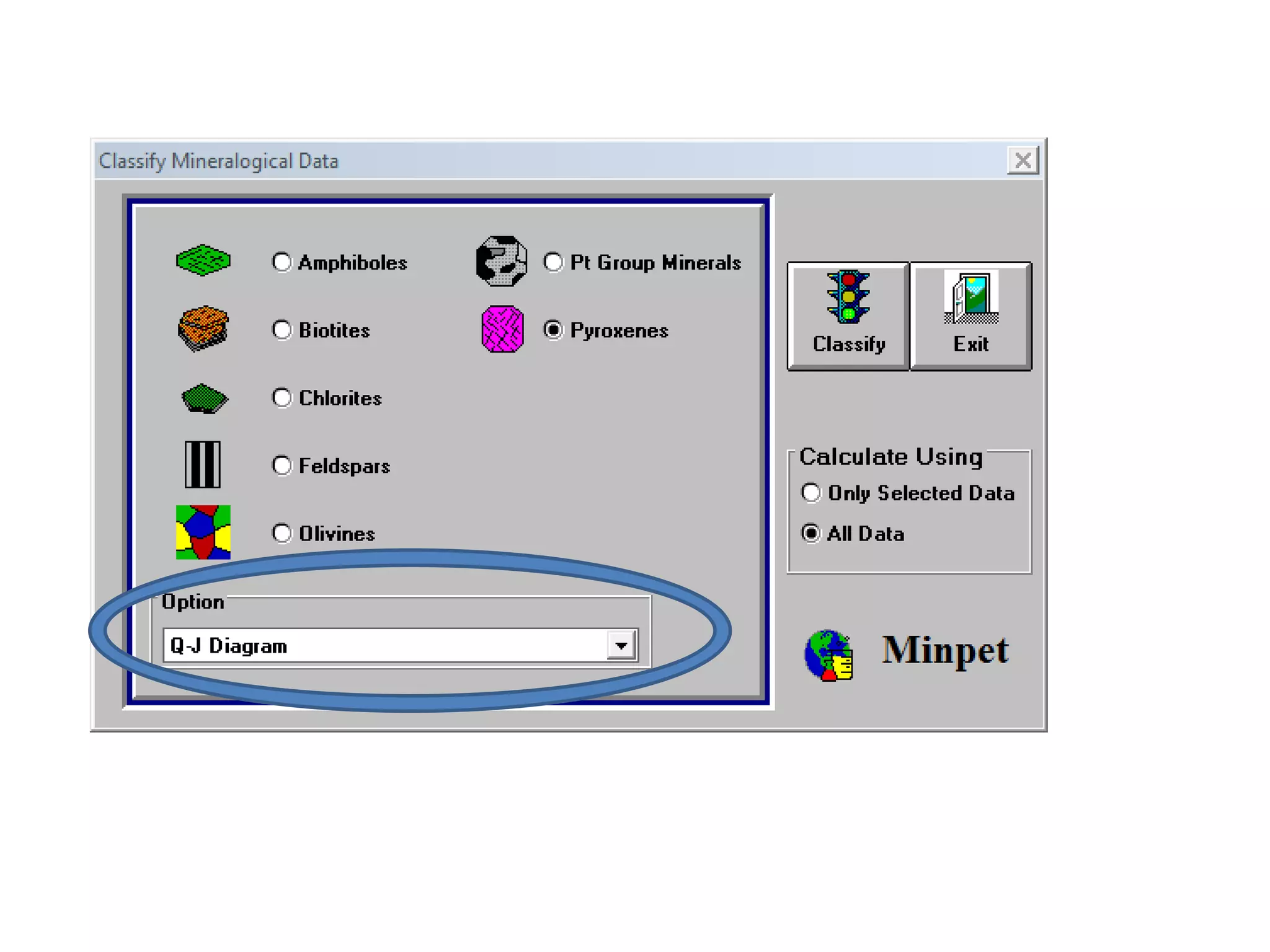
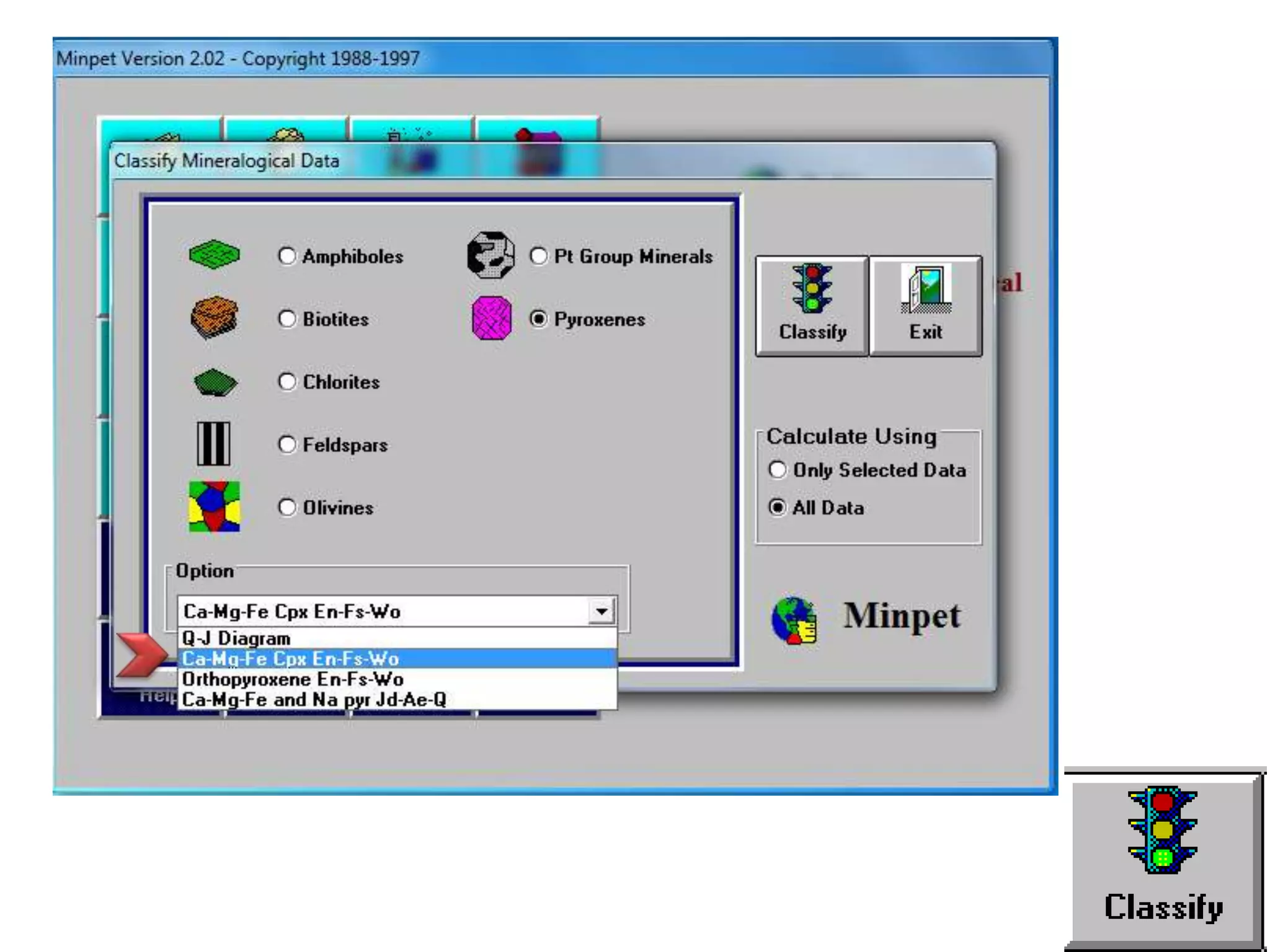
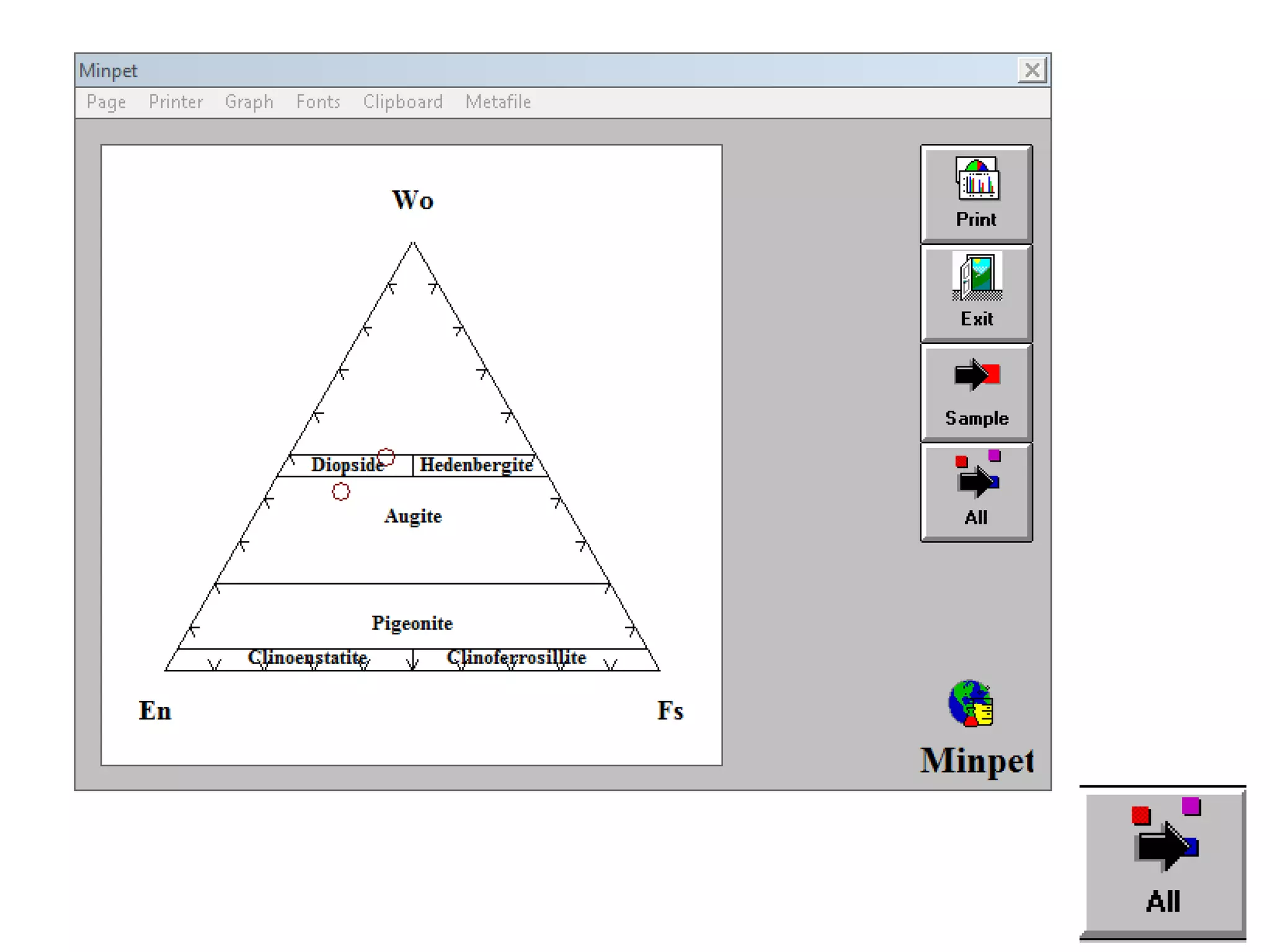
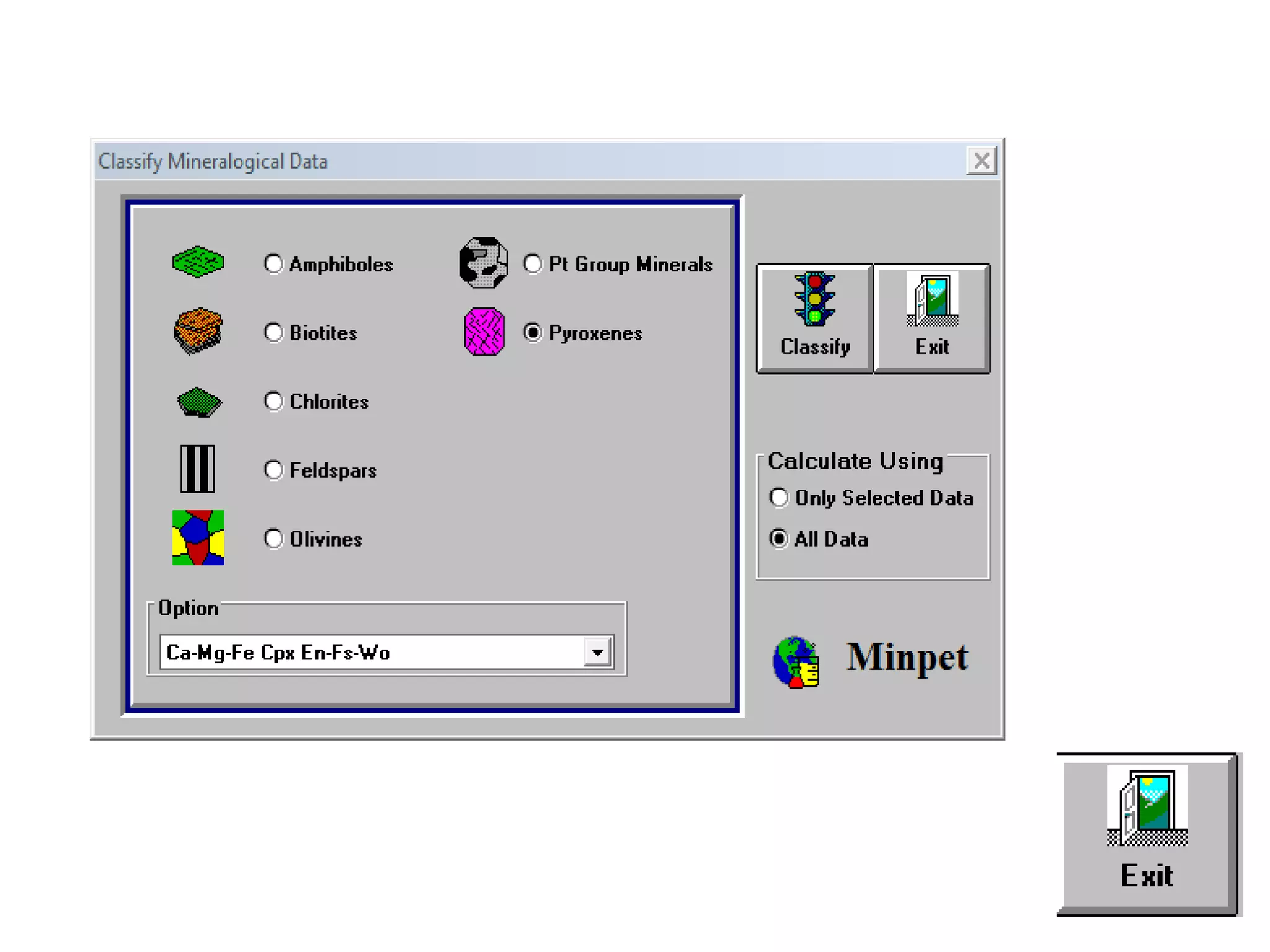
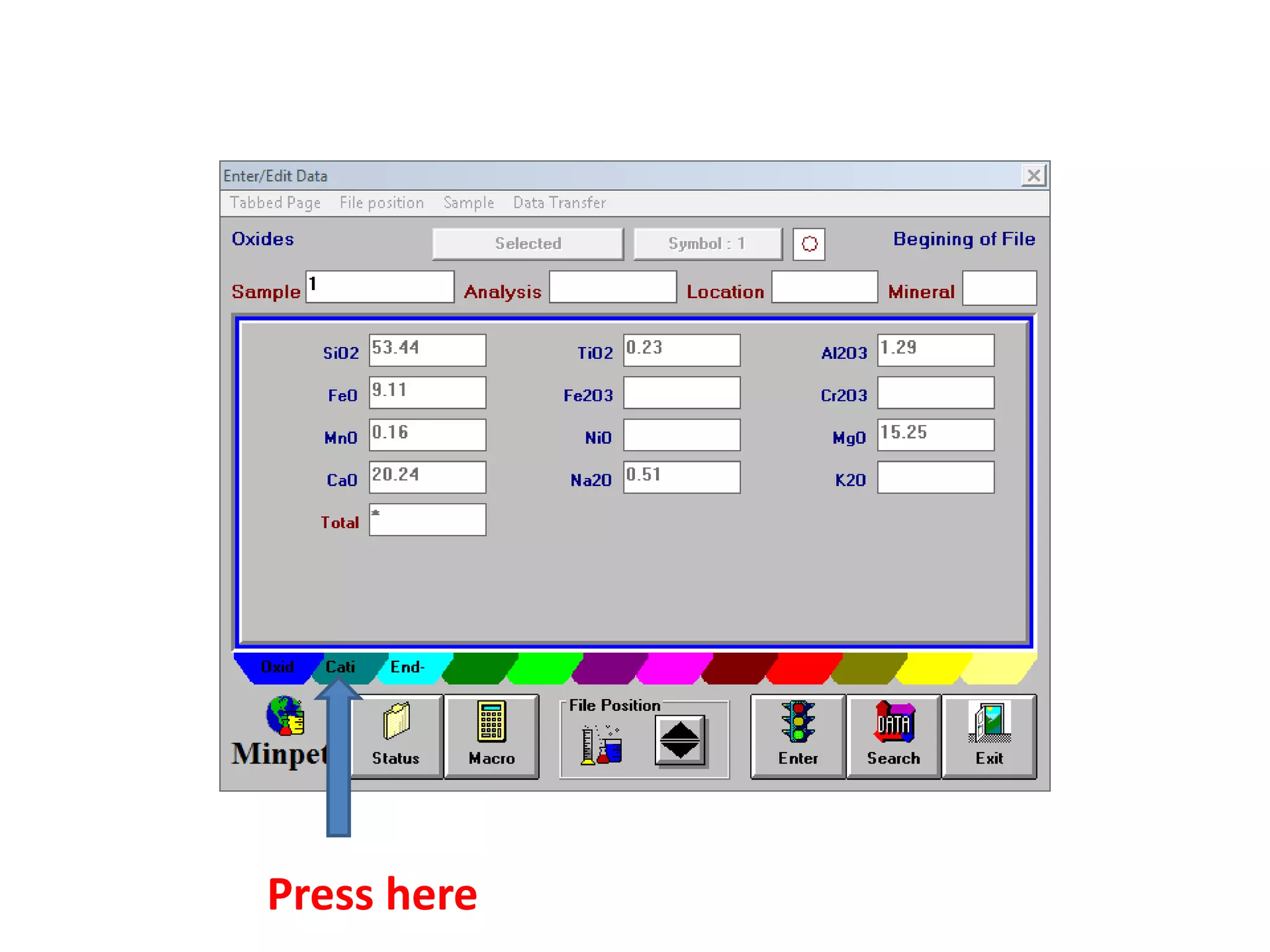
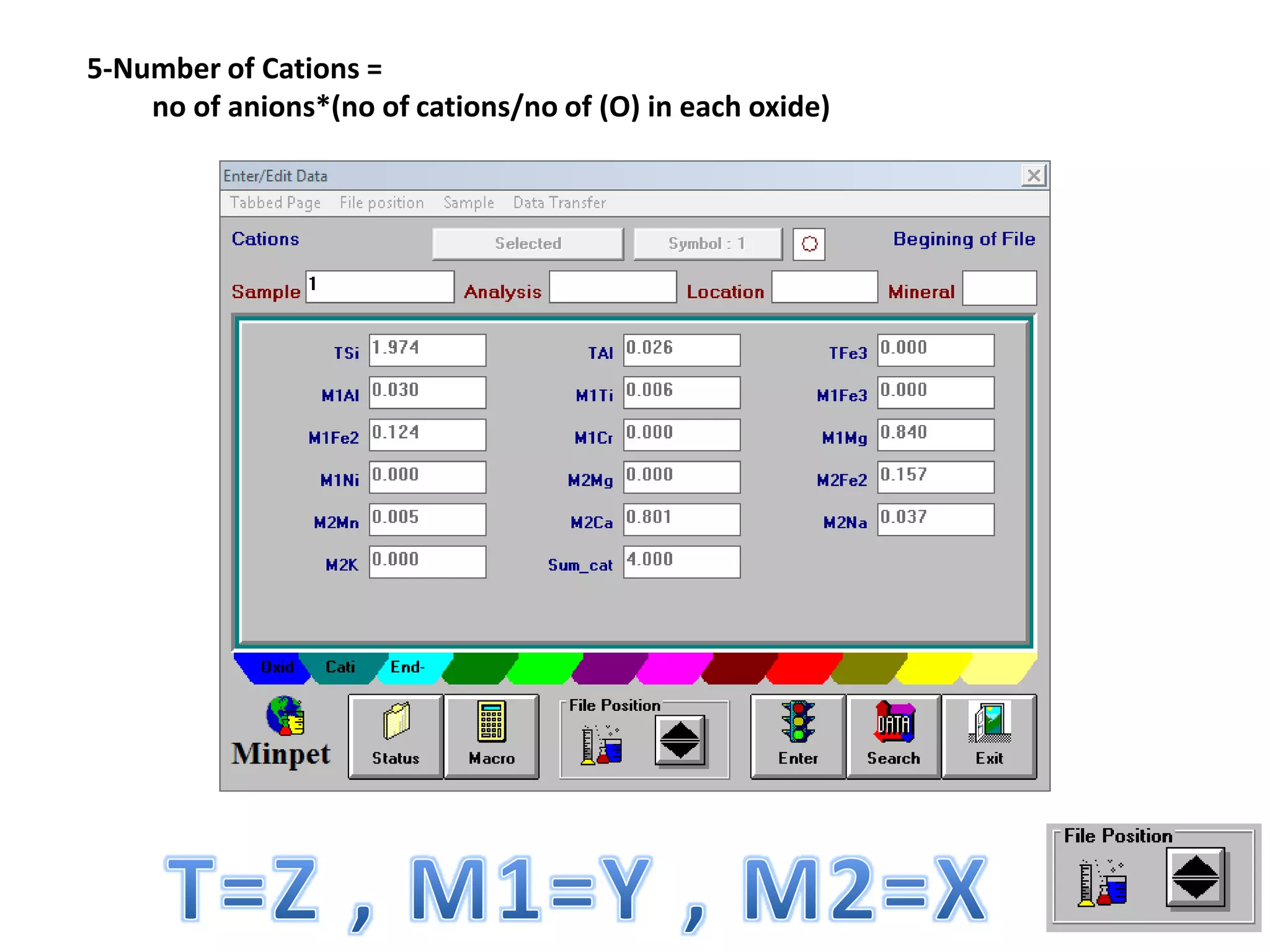
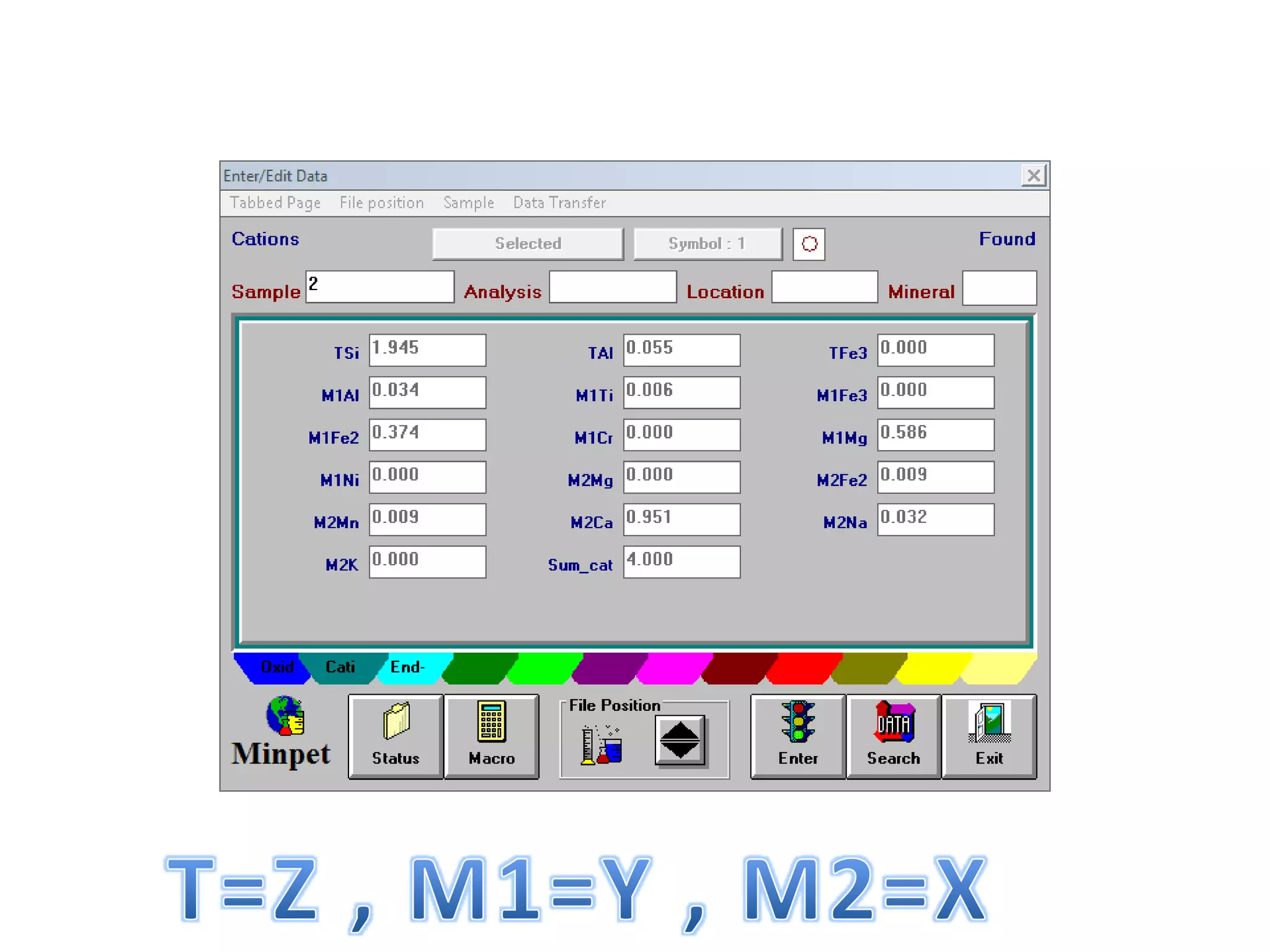
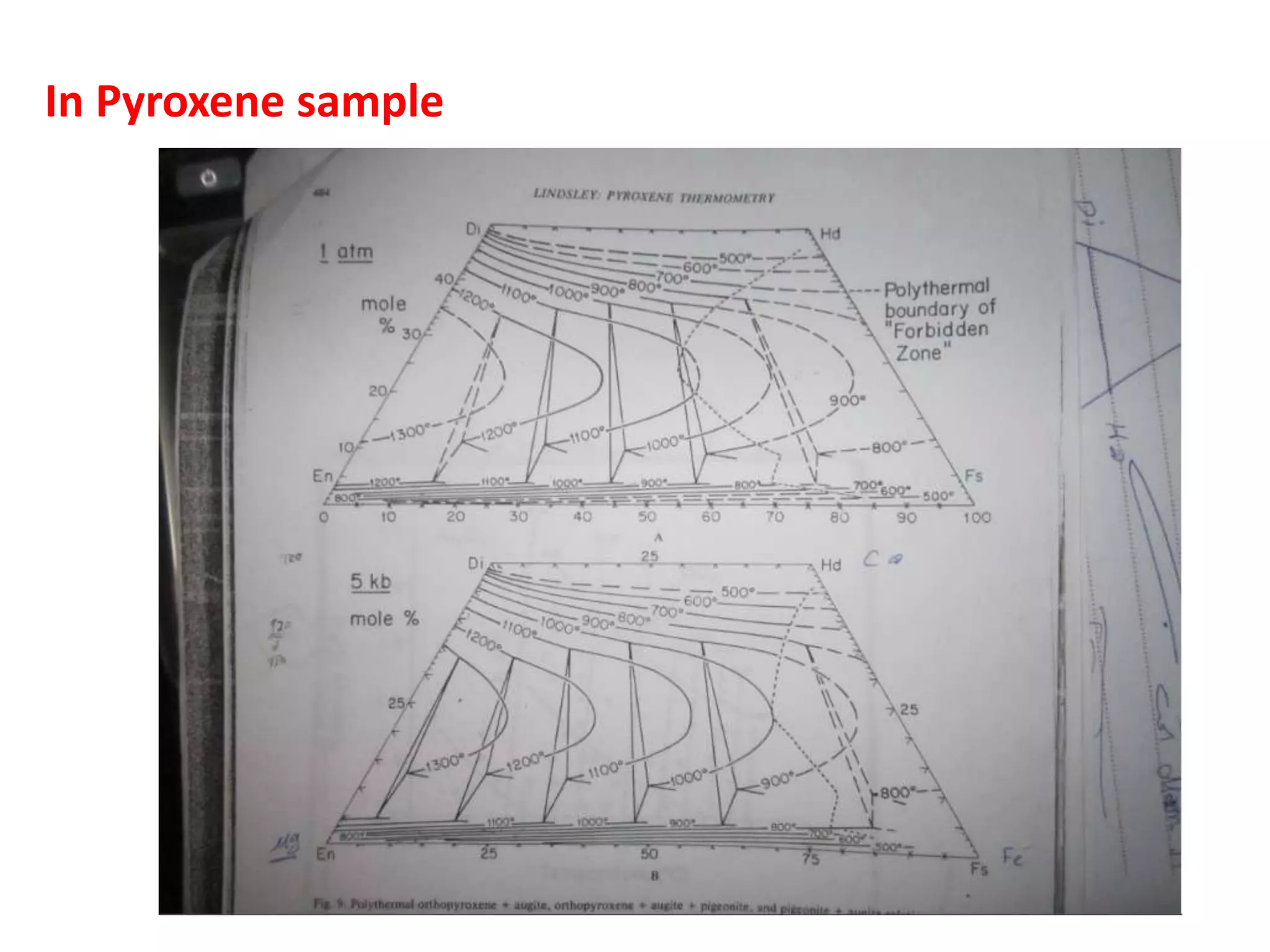
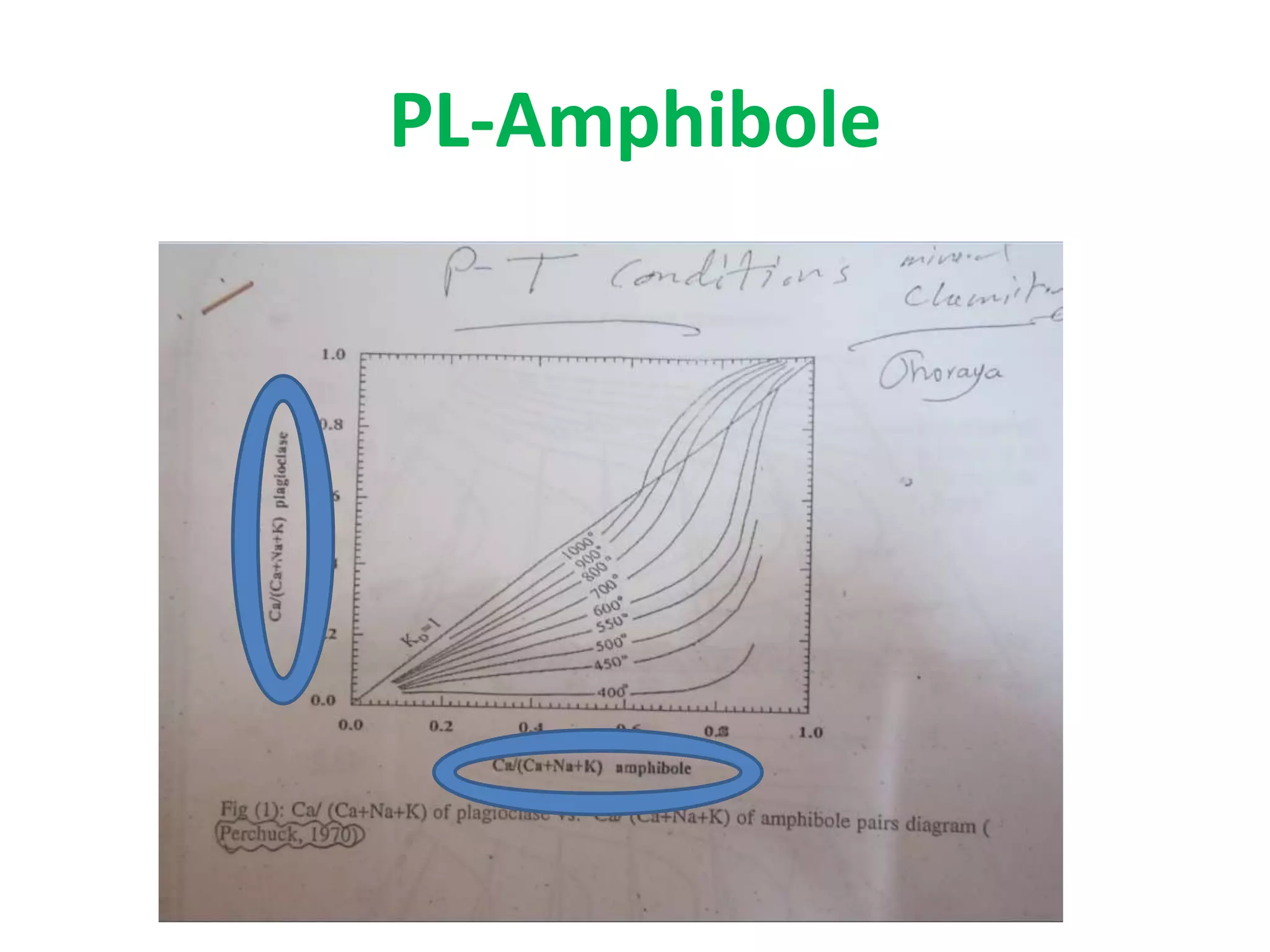
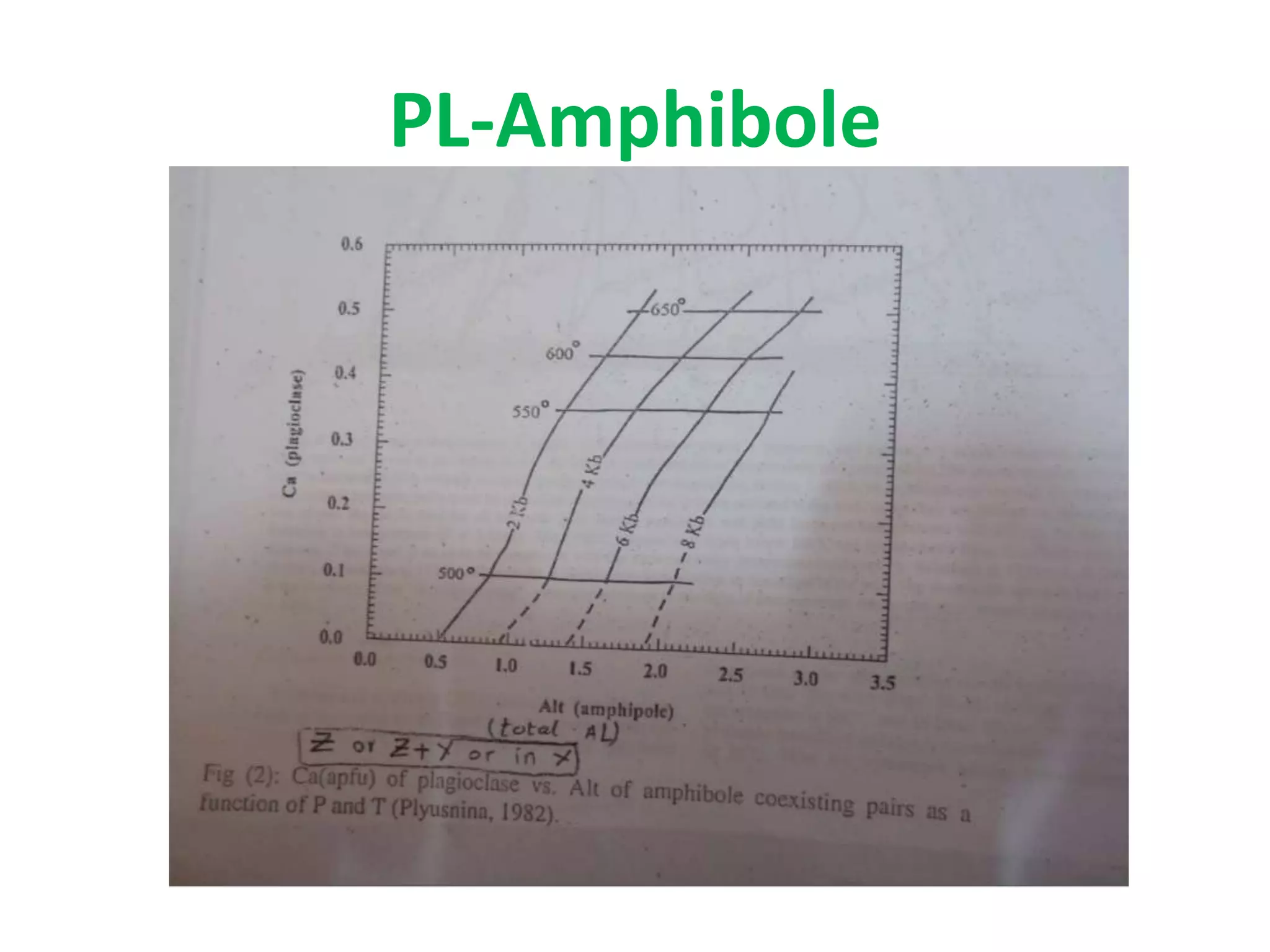
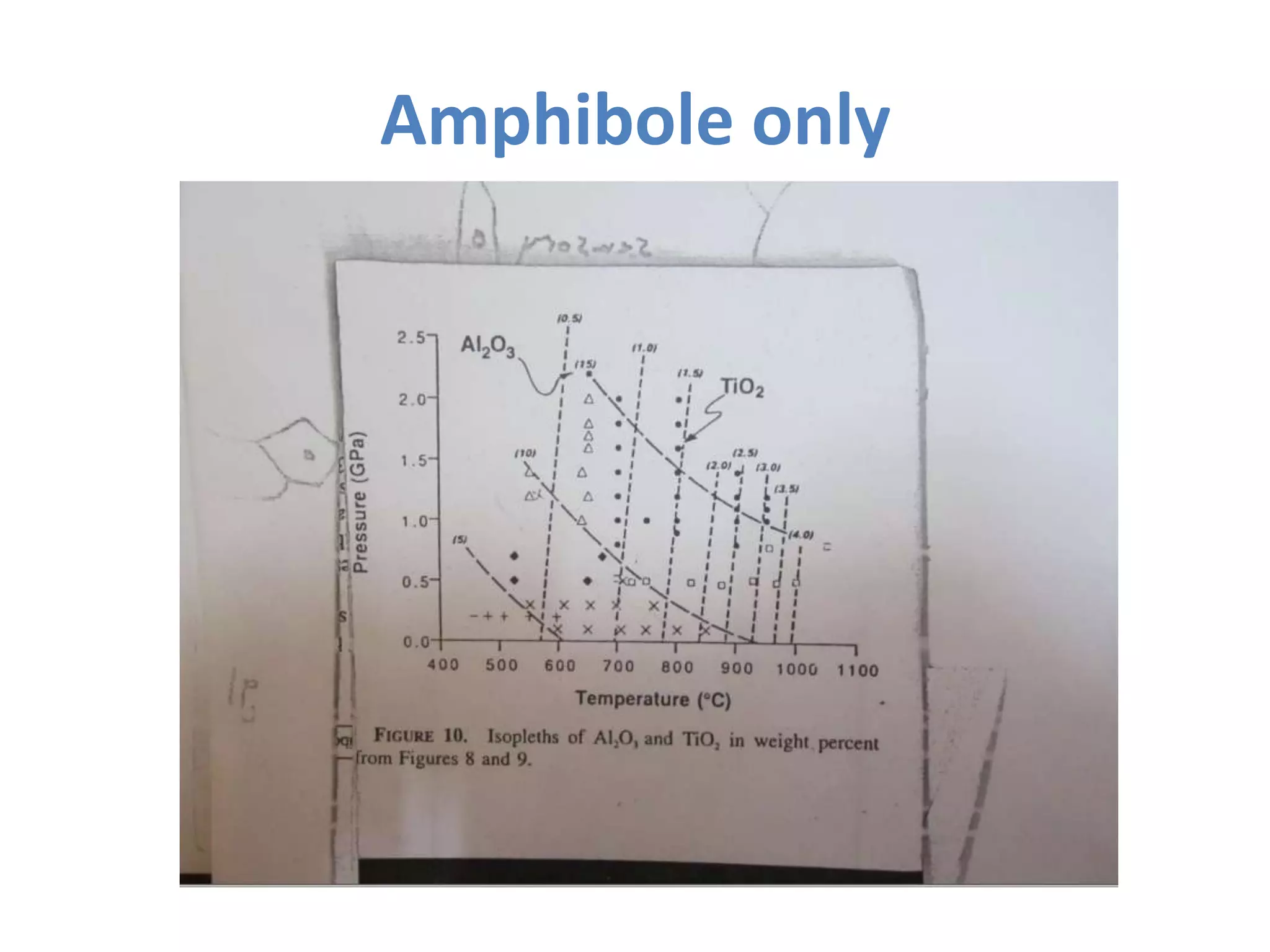
This document discusses mineral isotopes and chemistry from the Department of Geology at Tanta University in Egypt. It was submitted by Mohamed Mahmoud Ahmed El-Shora and covers topics including radioactivity, stable and unstable isotopes, geochronology using isotope dating, rare earth elements in minerals, and partial melting. Diagrams are included showing isotope ratios and rare earth element patterns in primitive mantle and residual and melt fractions during mantle melting. Methods for calculating mineral formulas based on oxide percentages are also outlined.