IB Chemistry on Redox, Oxidizing, Reducing Agents and writing half redox equations
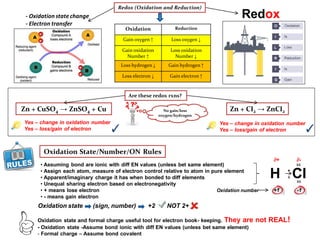
- 1. Redox- Oxidation statechange - Electron transfer Zn + CuSO4 → ZnSO4 + Cu Zn + CI2 → ZnCI2No gain/loss oxygen/hydrogen Are these redox rxns? Yes – change in oxidation number Yes – loss/gain of electron Yes – change in oxidation number Yes – loss/gain of electron✓ ✓ • Assuming bond are ionic with diff EN values (unless bet same element) • Assign each atom, measure of electron control relative to atom in pure element • Apparent/imaginary charge it has when bonded to diff elements • Unequal sharing electron based on electronegativity • + means lose electron • - means gain electron Oxidation State/Number/ON Rules Oxidation Reduction Gain oxygen ↑ Loss oxygen ↓ Gain oxidation Number ↑ Loss oxidation Number ↓ Loss hydrogen ↓ Gain hydrogen ↑ Loss electron ↓ Gain electron ↑ H CI xx xx • x ∂-∂+ +1 -1Oxidation number Oxidation state (sign, number) +2 NOT 2+ Oxidation state and formal charge useful tool for electron book- keeping. They are not REAL! - Oxidation state -Assume bond ionic with diff EN values (unless bet same element) - Formal charge – Assume bond covalent Redox (Oxidation and Reduction)
- 2. Redox (Oxidationand Reduction) • Assuming bond are ionic with diff EN values (unless bet same element) • Assign each atom, measure of electron control relative to atom in pure element • Apparent/imaginary charge it has when bonded to diff elements • Unequal sharing electron based on electronegativity • + means lose electron • - means gain electron Oxidation Number/ON Rules H CI xx xx • x ∂-∂+ +1 -1Oxidation number Oxidation state (sign, number) +2 NOT 2+ Oxidation state and formal charge useful tool for electron book- keeping. They are not REAL! - Oxidation state -Assume bond ionic with diff EN values (unless bet same element) - Formal charge – Assume bond covalent xx CI CI Oxidation Number/ON Rules H CI Na CI Imagine electron move to more EN element O C O oo o o xxoo oo xx xx o x Equal sharing xx xx xx o x +1 -1 Unequal sharing 0 0 xx xx xx o x +1 -1 Complete transfer ox ox o o oo oo oo oo o o xo xo Unequal sharing -2 +4 -2 H O H CI2 H CI Na CI C O2 +1 -1 -1+1 +4 -2 oo o x o x +1 -2 +1 Unequal sharing H N H H o x o x o x oo +1 -3 +1 +1 Unequal sharing H2 O N H3 H C H H H o x o xo x o x +1 +1 +1 +1 -4 C H4 +1 -2 -3 +1 -4 +1 CI C CI CI CI -1 -1 -1 -1 xx xx xx xx xx xx xx xx xx xx xx xx xo xo ox ox +4 C CI4 +4 -1 0
- 3. Exceptions Element ON Exception Example Hydrogen +1 H +1 -1 Bond to metal Metal hydride NaH CaH2 Oxidation Number/States/ON Rules Imagine electron move to more EN element • Assuming bond are ionic with diff EN values (unless bet same element) • Assign each atom, measure of electron control relative to atom in pure element • Apparent/imaginary charge it has when bonded to diff elements • Unequal sharing electron based on electronegativity H CI xx ∂- xx • x Oxidation number +1 -1 ∂+ xx H CI xx xx xx +1 -1 o x Unequal sharing H CI +1 -1 Na Ho x +1 -1 Complete transfer Na H +1 -1 Exceptions Element ON Exception Example Oxygen -2 O -2 +2 Bond to fluorine F2O Exceptions Element ON Exception Example Oxygen -2 O -2 -1 Peroxide (O-O) H2O2 H O H F O F oo oo o x o x +1 -2 +1 Unequal sharing H2 O +1 -2 oo oo xx xx xx xx xx xx ox ox -1 +2 -1 Unequal sharing F2 O -1 +2 O O HH O-O single bond oo oo oo oo ox ox o o equal sharing +1 -1 -1 +1 Unequal sharing H2 O2 +1 -1 EN fluorine higher ↑EN oxygen higher ↑ Oxidation Number/ON Rules Imagine electron move to more EN element O O H O H Oxidation state O different – depend element bond with – different EN values ! F O F O O xx xx xx xx xx xx Equal sharing 0 0 O2 0 oo oo oo oo ox ox +1 -2 +1 Unequal sharing H2 O +1 -2 xx xx xx xx xxxx ox ox -1 +2 -1 F2 O -1 +2 H H oo oo oo oo o o ox ox +1 -1 -1 +1 H2 O2 +1 -1 Unequal sharing Unequal sharing
- 4. Oxidation Number/ON Rules Imagine electron move to more EN element • Assuming bond are ionic with diff EN values (unless bet same element) • Assign each atom, measure of electron control relative to atom in pure element • Apparent/imaginary charge it has when bonded to diff elements • Unequal sharing electron based on electronegativity H CI xx ∂- xx • x Oxidation number +1 -1 ∂+ xx Oxidation Number/ON Rules Imagine electron move to more EN element O O H O H Oxidation state O different – depend element bond with – different EN values ! F O F O O xx xx xx xx xx xx Equal sharing 0 0 O2 0 oo oo oo oo ox ox +1 -2 +1 Unequal sharing H2 O +1 -2 xx xx xx xx xxxx ox ox -1 +2 -1 F2 O -1 +2 H H oo oo oo oo o o ox ox +1 -1 -1 +1 H2 O2 +1 -1 Unequal sharing Unequal sharing Oxidation Number/ON Rules Imagine electron move to more EN element Oxidation state N different – depend element bond with – different EN values ! H N H H H H O N O H O N O N O N O O O O ox ox o x x x x x +1 -3 +1 +1 -2 [N H4 ] -3 +1 +1 Unequal sharing xx ox ox xo xo xx oo oo oo oo +1 -2 +3 -2 Unequal sharing H N O2 +1 +3 -2 O ox ox xo xo xx oo o o o o +1 -2 +5 -2 -2 Unequal sharing H N O3 +1 +5 -2 ox ox xx oo xx oo o o o o o o o o o o o o o o o o oo oo -2 -2 -2 -5 -5-2 N2 O5 Unequal sharing -5 +2 +1 +
- 5. Oxidation Number/ON Rules Atoms uncombined free element state = ON = 0 Ion form – ON same as charged on ion 1 2 Mg Mg2+ Na Na+ O2 S8 O2- 3 ON for element same as its most common ion/group ON metal from Gp 1 – 3 ON non metal Gp 5 - 7 Anion/Nonmetal Gp 5 Gp 6 Gp 7 Oxidation state Oxidation state Oxidation state - 3 - 2 - 1 N 3- O 2- F 1- P 3- S 2- CI 1- Cation/Metal Gp 1 Gp 2 Gp 3 Oxidation state Oxidation state Oxidation state +1 +2 +3 Na 1+ Mg 2+ Al 3+ K1+ Ca 2+ Ga 3+ 4 CI2 0 0 0 0 0 +1 +2 -2 -1 -2 CI- S2- ON for transition metal varies Transition metal ions Transition metals ions (variable oxidation states) Sc +3 Ti +2 +3 V +2 +3 Cr +2 +3 +6 Mn +2 +3 +6 +7 Fe +2 +3 Co +2 +3 Ni +2 Cu +1 +2 Zn +2 Sc 3+ Ti 2+ Ti 3+ V 2+ V 3+ Cr 2+ Cr 3+ Cr 6+ Mn 2+ Mn 3+ Mn 6+ Mn 7+ Fe 2+ Fe 3+ Co 2+ Co 3+ Ni 2+ Cu 1+ Cu 2+ Zn 2+ Oxidation number Diff ON Charge on ion Click here on oxidation rulesClick here view simple step Notes Sc3+ Charge on Sc Oxidation number +3 Oxidation state -+ 3+
- 6. ON all atoms in polyatomic ion add up to charge of polyatomic ion ON all atoms in neutral molecule add up to 0 CO3 2- SO4 2- H2SO4 CO2 5 Oxidation Number/ON Rules HNO3 (+1)2 + (+6) + (-2)4 = 0 +1 +6 -2 (+4) + (-2)2 = 0 (+1) + (+5) + (-2)3 = 0 +4 -2 +1 +5 -2 (+4) + (-2)3 = -2 +4 -2 (+6) + (-2)4 = -2 NO3 1- +6 -2 (+5) + (-2)3 = -1 +5 -2 7 ON atom/molecule of element = 0 (NOT combined) H2 CI2 O2 Fe Cu Mg 0 0 0 0 0 0 8 Monoatomic ion – ON same as charged on ion Ionic compound Charge ion Oxidation number MgF2 Mg 2+ F 1- Mg (+2) F (-1) NaCI Na 1+ CI 1- Na (+1) CI (-1) KBr K 1+ Br 1- K (+1) Br (-1) CaI2 Ca 2+ I 1- Ca (+2) I (-1) Li3N Li 1+ N 3- Li (+1) N (-3) Al2O3 Al 3+ O 2- AI (+3) O (-2) 9 Formula compound Charge Oxidation number Name using oxidation number FeO Fe 2+ or 2+ +2 Iron (II) oxide Fe2O3 Fe 3+ or 3+ +3 Iron (III) oxide Cu2O Cu 1+ or 1+ +1 Copper (I) oxide CuO Cu 2+ or 2+ +2 Copper (II) oxide MnO2 Mn 4+ or 4+ +4 Manganese (IV) oxide MnO4 - Mn 7+ or 7+ +7 Manganese (VII) oxide K2Cr2O7 Cr 6+ or 6+ +6 Potassium dichromate (VI) Cr2O3 Cr 3+ or 3+ +3 Chromium (III) oxide Click here view chemguide notes 6
- 7. Oxidation Number/ON Rules 9 Metal more than one oxidation states, Roman numeral used Manganese Chromium Ionic compound MnSO4 MnO2 K2MnO4 KMnO4 K2Cr2O7 Cr2O3 Oxidation Number (+2) + (+6) + (-2)4 = 0 Mn (+2) (+4) + (-2)2 = 0 Mn (+4) (+1)2 + (+6) + (-2)4 = 0 Mn (+6) (+1) + (+7) + (-2)4 = 0 Mn (+7) (+1)2 + (+6)2 + (-2)7 = 0 Cr (VI) (+3)2 + (-2)3 = 0 Cr (III) IUPAC name Manganese (II) sulphate Manganese (IV) oxide Manganese (VI) Manganese (VII) Chromium (VI) Chromium (III) Iron Copper Ionic compound FeCI2 FeCI3 CuCI CuCI2 Oxidation Number (+2) + (-1)2 = 0 Fe (+2) (+3) + (-1)3 = 0 Fe (+3) (+1) + (-1) = 0 Cu (+1) (+2) + (-1)2 = 0 Cu (+2) IUPAC name Iron (II) chloride Iron (III) chloride Copper (I) chloride Copper (II) chloride Vanadium VO2 + VO 2+ (+5) + (-2)2 = +1 V (+5) (+4) + (-2) = +2 V (+4) Vanadium (V) Vanadium (IV) ON for underlined element in ionic compound10 Na2SO3 Na2SO4 NaNO2 NaNO3 (SO3)2- (SO4)2- (+1)2 + (+4) + (-2)3 = 0 (+1)2 + (+6) + (-2)4 = 0 (+1)+ (+3) + (-2)2 = 0 (+1)+ (+5) + (-2)3 = 0 (+4) + (-2)3 = -2 (+6) + (-2)4 = -2 ON for S = +4 ON for S = +6 ON for N = +3 ON for N = +5 ON for S = +4 ON for S = +6 +1 +4 -2 +1 +6 -2 +1 +3 -2 +1 +5 -2 +4 -2 +6 -2
- 8. Oxidation Number/ON Rules 11 ON for underlined element in compound OH-1 PO4 3- S2O3 2- CN-1 OCI-1 H2O2 (HCO3)-1 (-2) + (+1) = -1 (+5) + (-2)4 = -3 (+2)2 + (-2)3 = -2 (+4) + (-5) = -1 (-2) + (+1) = -1 (+1)2 + (-1)2 = 0 ON for O = -2 ON for P = +5 ON for S = +2 ON for C = +4 ON for O = -2 ON for O = -1 ON for C = +4 (+1) + (+4) + (-2)3 = -1 -2 +1 +5 -2 +2 -2 +4 -5 -2 +1 +1 -1 +1 +4 -2 Oxidation Reduction Gain oxygen ↑ Loss oxygen ↓ Loss hydrogen ↓ Gain hydrogen ↑ Redox (Oxidationand Reduction) Rxn involve gain/loss of oxygen/hydrogen CH4 + 2O2 → CO2 + 2H2O Gain hydrogen Oxygen reduced gain oxygen Carbon oxidation Rxn involve gain/loss of electron Oxidation Reduction Gain ON ↑ Loss ON ↓ Loss electron ↓ Gain electron ↑ - broader definition - cover more rxn types PbO + CO → Pb + CO2 Lead Reduction gain oxygen Carbon oxidized (-4) (+4) (0) (-2) ON ↑ ON ↓ oxygen reduced carbon oxidized PbO + CO → Pb + CO2 (+2) (0) lead reduced (+2) (+4) CH4 + 2O2 → CO2 + 2H2O ON ↑ carbon oxidized ON ↓ loss oxygen
- 9. Redox (Oxidation and Reduction) Oxidation – Gain of oxygen ↑ Oxidation – Loss of hydrogen ↓ Reduction – Gain of hydrogen ↑ Reduction – Loss of oxygen ↓ Oxidation Reduction Gain oxygen ↑ Loss oxygen ↓ Gain oxidation Number ↑ Loss oxidation Number ↓ Loss hydrogen↓ Gain hydrogen ↑ Loss electron ↓ Gain electron ↑ Ca + O2 → CaO CH4 + 2O2 → CO2+ 2H2O gain oxygen gain oxygen Zn + CuO → ZnO + Cu PbO + CO → Pb + CO2 loss oxygen loss oxygen H2S + CI2 → S +2HCI loss hydrogen H2S + CI2 → S + 2HCI Redox- Oxidation state change - Electron transfer CH4 + 2O2 → CO2 + 2H2O gain hydrogen Zn + CuSO4 → ZnSO4 + Cu Zn + CI2 → ZnCI2 No gain/loss oxygen/hydrogen Redox gain oxygen gain hydrogen Reduction Oxidation Are these redox rxns? Most rxn does not involve H2 and O2
- 10. carbon oxidized Oxidation Reduction Gain oxygen ↑ Loss oxygen ↓ Loss hydrogen ↓ Gain hydrogen ↑ Redox (Oxidationand Reduction) Rxn involve gain/loss of oxygen/hydrogen CH4 + 2O2 → CO2 + 2H2O Gain hydrogen oxygen reduced gain oxygen carbon oxidized Rxn involve gain/loss of electron Oxidation Reduction Gain ON ↑ Loss ON ↓ Loss electron ↓ Gain electron ↑- broader definition - cover more rxn types lead reduced gain oxygen carbon oxidized (-4) (+4) (0) (-2) ON ↑ ON ↓ oxygen reduced carbon oxidized (+2) (0) lead reduced (+2) (+4) CH4 + 2O2 → CO2 + 2H2O ON ↑ ON ↓ loss oxygen PbO + CO → Pb + CO2PbO + CO → Pb + CO2 Oxidizing Agent Reducing Agent Causes Oxidation Cause Reduction Undergo reduction Undergo oxidation Gain electron ↑ Loss electron ↓ Decrease oxidation number ↓ Increase oxidation number ↑ Oxidation Reduction Gain oxygen ↑ Loss oxygen ↓ Gain oxidation Number ↑ Loss oxidation Number ↓ Loss hydrogen ↓ Gain hydrogen ↑ Loss electron ↓ Gain electron ↑ Oxidizing Agent Reducing Agent MnO4 - Fe2+ Cr2O7 2- SO2 HNO3 I- H2O2 H2S CI2 SO3 2-
- 11. CI2 + 2KBr-→ 2KCI + Br2 3CuO + 2NH3→ 3H2O+ 3Cu + N2 Redox (Oxidationand Reduction) (+7) (+2)Mn red - ON ↓ (+2) Fe oxi – ON ↑ (+3) MnO4 - + Fe2+ + 8H+ → Mn2+ + Fe3+ 4H2O Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation Oxidizing Agent Reducing Agent MnO4 - Fe2+ Reduction Oxidation Oxidizing Agent Reducing Agent CI2 Br- Reduction Oxidation Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation (0) CI red – ON ↓ (-1) (-1) Br - oxi – ON ↑ (0) Oxidizing Agent Reducing Agent CuO NH3 Reduction Oxidation Reducing agent ↓ Oxidation (-3) NH3 oxi – ON ↑ (0) Oxidizing agent ↓ Reduction (+2) Cu red – ON ↓ (0) 2HCI + Zn → H2 + ZnCI2 (0) Zn oxi – ON ↑ (+2)Reducing agent ↓ Oxidation Oxidizing agent ↓ Reduction (+1) H red – ON ↓ (0) Oxidizing Agent Reducing Agent HCI Zn Reduction Oxidation
- 12. CI2 + 2KBr-→ 2KCI + Br2 3CuO + 2NH3→ 3H2O+ 3Cu +N2 Redox (Oxidationand Reduction) (+7) (+2)Mn red - ON ↓ (+2) Fe oxi – ON ↑ (+3) MnO4 - + 8H+ + Fe2+ → Mn2+ + Fe3+ 4H2O Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation Oxidizing Agent Reduction MnO4 - + 5e → Mn2+ Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation (0) CI red – ON ↓ (-1) (-1) Br - oxi – ON ↑ (0) Reducing agent ↓ Oxidation (-3) NH3 oxi – ON ↑ (0) Oxidizing agent ↓ Reduction (+2) Cu red – ON ↓ (0) 2HCI + Zn → H2 + ZnCI2 (0) Zn oxi – ON ↑ (+2)Reducing agent ↓ Oxidation Oxidizing agent ↓ Reduction (+1) H red – ON ↓ (0) Reducing Agent Oxidation Fe 2+ → Fe2+ + e- Loss electron Increase ON ↑ Gain electron Decrease ON ↓ Reducing Agent Oxidation 2Br - → Br2 + 2e- Loss electron Increase ON ↑ Oxidizing Agent Reduction CI2 + 2e → 2CI- Gain electron Decrease ON ↓ Reducing Agent Oxidation (NH3) -N3- → N + 3e- Loss electron Increase ON ↑ Oxidizing Agent Reduction (CuO) Cu2+ + 2e → Cu Gain electron Decrease ON ↓ Reducing Agent Oxidation Zn → Zn2+ + 2e- Loss electron Increase ON ↑ Oxidizing Agent Reduction 2H+ + 2e → H2 Gain electron Decrease ON ↓
- 13. Redox (Oxidationand Reduction) Half equations Oxidation rxn Oxidation half eqn Reduction half eqn Loss electron ↓ Reduction rxn Loss hydrogen ↓ Gain oxygen ↑ Gain ON ↑ Gain electron ↑ Gain hydrogen ↑ Loss oxygen ↓ Loss ON ↓ OxidizingAgentReducing Agent Oxidation rxn Reduction rxn lose electron Zn + 2H+ → H2 + Zn2+ Zn → Zn2+ + 2e 2H+ + 2e → H2 (0) ON increase ↑ (+2) Zn → Zn2+ + 2e 2H+ + 2e → H2 2H+ + Zn → Zn2+ + H2 lose electron gain electron (+1) ON decrease ↓ (0) Completefull eqn Zn + Cu2+ → Zn2+ + CuOxidation half eqn Zn → Zn2+ + 2e lose electron (0) ON increase ↑ (+2) Reduction half eqn Cu2+ + 2e → Cu (+2) ON decrease ↓ (0) gain electron Zn → Zn2+ + 2e Cu2+ + 2e → Cu Cu2+ + Zn → Zn2+ + Cu Half equations
- 14. Redox (Oxidationand Reduction) Half equations Oxidation half eqn Reduction half eqn Zn → Zn2+ + 2e 2H+ + 2e → H2 (0) ON increase ↑ (+2) Zn → Zn2+ + 2e 2H+ + 2e → H2 2H+ + Zn → Zn2+ + H2 lose electron gain electron (+1) ON decrease ↓ (0) Completefull eqn Oxidation half eqn Zn → Zn2+ + 2e lose electron (0) ON increase ↑ (+2) Reduction half eqn Cu2+ + 2e → Cu (+2) ON decrease ↓ (0) gain electron Zn → Zn2+ + 2e Cu2+ + 2e → Cu Cu2+ + Zn → Zn2+ + Cu Half equations Zn + 2HCI → H2 + ZnCI2 Zn + 2H+ + 2CI- → H2 + Zn2+ + 2CI - Completeionic/redox eqn Zn + 2H+ → H2 + Zn2+ spectator ionsspectator ions Zn + 2H+ → H2 + Zn2+ Zn + CuSO4 → ZnSO4 + Cu Zn + Cu2++ SO4 2- → Zn2+ + SO4 2- + Cu Completefull eqn Completeionic/redox eqn spectator ions Zn + Cu2+ → Zn2+ + Cu Half equations Half equations Zn + Cu2+ → Zn2+ + Cu
- 15. Redox (Oxidationand Reduction) Half equations Oxidation half eqn Reduction half eqn Mg → Mg2+ + 2e Pb2+ + 2e → Pb (0) ON increase ↑ (+2) Mg → Mg2+ + 2e Pb2+ + 2e → Pb Pb2+ + Mg → Mg2+ + Pb lose electron gain electron (+2) ON decrease ↓ (0) Completefull eqn Oxidation half eqn 2Br- → Br2 + 2e lose electron (-1) ON increase ↑ (0) Reduction half eqn CI2 + 2e → 2CI- (0) ON decrease ↓ (-1) gain electron 2Br- → Br2 + 2e CI2 + 2e → 2CI- CI2 + 2Br- → 2CI- + Br2 Half equations Mg + PbO → Pb + MgO Mg + Pb2+ + O2- → Pb + Mg2+ + O 2- Completeionic/redox eqn spectator ionsspectator ions Mg + Pb2+ → Pb + Mg2+ 2KBr + CI2 → Br2 + 2KCI 2K+ + 2Br- + CI2 → Br2 + 2K+ + 2CI - Completefull eqn Completeionic/redox eqn spectator ions 2Br- + CI2 → Br2 + 2CI- Half equations Half equations Mg + Pb2+ → Pb + Mg2+ 2Br- + CI2 → Br2 + 2CI- lose electron
- 16. MnO4 - + 8H+ + 5Fe2+ → Mn2+ + 5Fe3+ + 4H2O ConstructingHalf and complete redox equation (+7) (+2)Mn red - ON ↓ (+2) Fe oxi – ON ↑ (+3) MnO4 - + Fe2+ + 8H+ → Mn2+ + Fe3+ + 4H2O Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation Oxidizing Agent Reduction MnO4 - + 5e → Mn2+ Reducing Agent Oxidation Fe 2+ → Fe2+ + e- Loss electron Increase ON ↑ Gain electron Decrease ON ↓ Completefull eqn Oxidation half eqnReduction half eqn 1. Balance # O -add H2O 2. Balance# H add H+ 3. Balance# charges -add electrons 4. Balance# electron transfer MnO4 - → Mn2+ MnO4 - → Mn2+ + 4H2O MnO4 - + 8H+ → Mn2++ 4H2O MnO4 - + 8H+ + 5e- → Mn2+ + 4H2O Fe2+ → Fe3+ Fe2+ → Fe3+ + e- 5Fe2+ → 5Fe3+ + 5e-MnO4 - + 8H+ + 5e- → Mn2+ + 4H2O x 5x 1 MnO4 - + 8H+ + 5e- → Mn2+ + 4H2O 5Fe2+ → 5Fe3+ + 5e- + MnO4 - - In acidic medium - Strong oxidizing agent MnO4 - + 8H+ + Fe2+ → Mn2+ + Fe3+ 4H2O
- 17. 2MnO4 - + 5SO2+ 2H2O → 2Mn2+ + 5SO4 2- + 4H+ ConstructingHalf and complete redox equation (+7) (+2)Mn red - ON ↓ (+4) SO2 oxi – ON ↑ (+6) 2MnO4 - + 5SO2 + 2H2O→ 2Mn2+ + 5SO4 2- + 4H+ Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation Oxidizing Agent Reduction MnO4 - + 5e → Mn2+ Reducing Agent Oxidation SO2 → SO4 2- + 2e- Loss electron Increase ON ↑ Gain electron Decrease ON ↓ Completefull eqn Oxidation half eqnReduction half eqn 1. Balance # O - add H2O 2. Balance# H add H+ 3. Balance# charges - add electrons 4. Balance# electron transfer MnO4 - → Mn2+ MnO4 - → Mn2+ + 4H2O MnO4 - + 8H+ → Mn2++ 4H2O MnO4 - + 8H+ + 5e- → Mn2+ + 4H2O SO2 → SO4 2- 2MnO4 - + 16H+ + 10e- → 2Mn2+ + 8H2O x 5x 2 2MnO4 - + 16H+ + 10e- → 2Mn2+ + 8H2O 5SO2 + 10H2O → 5SO4 2- + 20H+ + 10e- + 2MnO4 - + 5SO2 + 2H2O→ 2Mn2+ + 5SO4 2- 4H+ SO2 + 2H2O → SO4 2- SO2 + 2H2O → SO4 2- + 4H+ SO2 + 2H2O → SO4 2- + 4H+ + 2e- 5SO2 + 10H2O → 5SO4 2- + 20H+ + 10e-
- 18. 2MnO4 - + 5H2O2 + 6H+ → 2Mn2+ + 5O2 + 8H2O ConstructingHalf and complete redox equations (+7) (+2)Mn red - ON ↓ (-1) H2O2 oxi – ON ↑ (0) 2MnO4 - + 5H2O2 + 6H+ → 2Mn2+ + 5O2 + 8H2O Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation Oxidizing Agent Reduction MnO4 - + 5e → Mn2+ Reducing Agent Oxidation H2O2 → O2 + 2e- Loss electron Increase ON ↑ Gain electron Decrease ON ↓ Completefull eqn Oxidation half eqnReduction half eqn 1. Balance # O - add H2O 2. Balance# H add H+ 3. Balance# charges - add electrons 4. Balance# electron transfer MnO4 - → Mn2+ MnO4 - → Mn2+ + 4H2O MnO4 - + 8H+ → Mn2++ 4H2O MnO4 - + 8H+ + 5e- → Mn2+ + 4H2O 2MnO4 - + 16H+ + 10e- → 2Mn2+ + 8H2O x 5x 2 2MnO4 - + 16H+ + 10e- → 2Mn2+ + 8H2O 5H2O2 → 5O2 + 10H+ + 10e- + 2MnO4 - + 5H2O2 + 6H+ → 2Mn2+ + 5O2 + 8H2O H2O2 → O2 H2O2 → O2 + 2H+ H2O2 → O2 + 2H+ + 2e- 5H2O2 → 5O2 + 10H+ + 10e-
- 19. Cr2O7 2- + 3NO2 - + 8H+ → 2Cr3+ + 3NO3 - + 4H2O Cr2O7 2-→ 2Cr3+ ConstructingHalf and complete redox equations (+6) (+3)Cr red - ON ↓ (+3) NO2 - oxi – ON ↑ (+5) Cr2O7 2- + 3NO2 - + 8H+ → 2Cr3+ + 3NO3 - + 4H2O Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation Oxidizing Agent Reduction Cr2O7 2- + 6e- → 2Cr3+ Reducing Agent Oxidation NO2 - → NO3 - + 2e- Loss electron Increase ON ↑ Gain electron Decrease ON ↓ Completefull eqn Oxidation half eqnReduction half eqn 1. Balance # O - add H2O 2. Balance# H add H+ 3. Balance# charges - add electrons 4. Balance# electron transfer x 3x 1 Cr2O7 2- + 14H+ + 6e- → 2Cr3+ + 7H2O 3NO2 -+ 3H2O → 3NO3 - + 6H+ + 6e- + Cr2O7 2- + 3NO2 - + 8H+ → 2Cr3+ + 3NO3 - + 4H2O Cr2O7 2- → 2Cr3+ + 7H2O Cr2O7 2- + 14H+ → 2Cr3+ + 7H2O Cr2O7 2- + 14H+ + 6e- → 2Cr3+ + 7H2O Cr2O7 2- + 14H+ + 6e- → 2Cr3+ + 7H2O NO2 - → NO3 - NO2 - + H2O → NO3 - NO2 - + H2O → NO3 - + 2H+ NO2 - + H2O → NO3 - + 2H+ + 2e- 3NO2 - + 3H2O → 3NO3 - + 6H+ + 6e-
- 20. Cr2O7 2- + 6Fe2+ + 14H+ → 2Cr3+ + 6Fe3+ + 7H2O Cr2O7 2-→ 2Cr3+ ConstructingHalf and complete redox equations (+6) (+3)Cr red - ON ↓ (+2) Fe2+ oxi – ON ↑ (+3) Cr2O7 2- + 6Fe2+ + 14H+ → 2Cr3+ + 6Fe3+ + 7H2O Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation Oxidizing Agent Reduction Cr2O7 2- + 6e- → 2Cr3+ Reducing Agent Oxidation Fe2+ → Fe3+ + e- Loss electron Increase ON ↑ Gain electron Decrease ON ↓ Completefull eqn Oxidation half eqnReduction half eqn 1. Balance # O - add H2O 2. Balance# H add H+ 3. Balance# charges - add electrons 4. Balance# electron transfer x 6x 1 Cr2O7 2- + 14H+ + 6e- → 2Cr3+ + 7H2O 6Fe2+ → 6Fe3+ + 6e- + Cr2O7 2- → 2Cr3+ + 7H2O Cr2O7 2- + 14H+ → 2Cr3+ + 7H2O Cr2O7 2- + 14H+ + 6e- → 2Cr3+ + 7H2O Cr2O7 2- + 14H+ + 6e- → 2Cr3+ + 7H2O Cr2O7 2- + 6Fe2+ + 14H+ → 2Cr3+ + 6Fe3+ 7H2O Fe2+ → Fe3+ Fe2+ → Fe3+ + e 6Fe2+ → 6Fe3+ + 6e
- 21. ConstructingHalf and complete redox equations (+5) (-1)CIO3 - red - ON ↓ (-1) I- oxi – ON ↑ (0) CIO3 - + 6I- + 6H+ → 3I2 + CI- + 3H2O Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation Oxidizing Agent Reduction CIO3 - + 6e- → CI- Reducing Agent Oxidation 2I- → I2 + 2e- Loss electron Increase ON ↑ Gain electron Decrease ON ↓ Completefull eqn Oxidation half eqnReduction half eqn 1. Balance # O - add H2O 2. Balance# H add H+ 3. Balance# charges - add electrons 4. Balance# electron transfer x 3x 1 CIO3 - + 6H+ + 6e- → CI- + 3H2O 6I- → 3I2 + 6e- + CIO3 - + 6I- + 6H+ → 3I2 + CI- + 3H2O CIO3 - → CI- CIO3 - → CI- + 3H2O CIO3 - + 6H+ → CI- + 3H2O CIO3 - + 6H+ + 6e- → CI- + 3H2O CIO3 - + 6H+ + 6e- → CI- + 3H2O 2I- → I2 2I- → I2 + 2e- 6I- → 3I2 + 6e- CIO3 - + 6H++ 6I- → 3I2 + 3H2O
- 22. ConstructingHalf and complete redox equations (+5) (+2)NO3 - red - ON ↓ (0) Cu oxi – ON ↑ (+2) 2NO3 - + 3Cu + 8H+ → 3Cu2+ + 2NO + 4H2O Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation Oxidizing Agent Reduction NO3 - + 3e- → NO Reducing Agent Oxidation Cu → Cu2+ + 2e- Loss electron Increase ON ↑ Gain electron Decrease ON ↓ Completefull eqn Oxidation half eqnReduction half eqn 1. Balance # O - add H2O 2. Balance# H add H+ 3. Balance# charges - add electrons 4. Balance# electron transfer x 3x 2 2NO3 - + 8H+ + 6e- → 2NO + 4H2O 3Cu → 3Cu2+ + 6e- + 2NO3 - + 3Cu + 8H+ → 3Cu2+ + 2NO + 4H2O NO3 - → NO NO3 - → NO + 2H2O NO3 - + 4H+ → NO + 2H2O NO3 - + 4H+ + 3e- → NO + 2H2O 2NO3 - + 8H+ + 6e- → 2NO + 4H2O Cu → Cu2+ Cu → Cu2+ + 2e- 3Cu → 3Cu2+ + 6e- 2NO3 - + 8H+ + 3Cu → 3Cu2+ +2NO + 4H2O
- 23. HNO3 +3Fe2+ + 3H+ → 3Fe3+ + NO + 2H2O ConstructingHalf and complete redox equations (+5) (+2)HNO3 red - ON ↓ (+2) Fe oxi – ON ↑ (+3) HNO3 + 3Fe2+ + 3H+ → 3Fe3+ + NO + 2H2O Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation Oxidizing Agent Reduction HNO3 + 3e- → NO Reducing Agent Oxidation Fe 2+ → Fe3++ e- Loss electron Increase ON ↑ Gain electron Decrease ON ↓ Completefull eqn Oxidation half eqnReduction half eqn 1. Balance # O - add H2O 2. Balance# H add H+ 3. Balance# charges - add electrons 4. Balance# electron transfer x 3x 1 HNO3 + 3H+ + 3e- → NO + 2H2O 3Fe2+ → 3Fe3+ + 3e- + HNO3 → NO + 2H2O HNO3+ 3H+ → NO + 2H2O HNO3 + 3H+ + 3e- → NO + 2H2O HNO3 + 3H+ + 3e- → NO + 2H2O Fe2+ → Fe3+ HNO3 + 3Fe2+ + 3H+ → 3Fe3+ + NO + 2H2O HNO3 → NO Fe2+ → Fe3+ + e- 3Fe2+ → 3Fe3+ + 3e-
- 24. H2O2 + 2Fe2+ +2H+ → 2Fe3+ + 2H2O ConstructingHalf and complete redox equations (-1) (-2)H2O3 red - ON ↓ (+2) Fe oxi – ON ↑ (+3) H2O2 + 2Fe2+ + 2H+ → 2Fe3+ + 2H2O Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation Oxidizing Agent Reduction H2O3 + e- → H2O Reducing Agent Oxidation Fe 2+ → Fe3++ e- Loss electron Increase ON ↑ Gain electron Decrease ON ↓ Completefull eqn Oxidation half eqnReduction half eqn 1. Balance # O - add H2O 2. Balance# H add H+ 3. Balance# charges - add electrons 4. Balance# electron transfer x 2x 1 H2O2 + 2H+ + 2e- → 2H2O 2Fe2+ → 2Fe3+ + 2e- + Fe2+ → Fe3+ Fe2+ → Fe3+ + e- 2Fe2+ → 2Fe3+ + 2e- H2O2 + 2Fe2+ + 2H+ → 2Fe3+ + 2H2O H2O2 → H2O H2O2 → 2H2O H2O2 + 2H+ → 2H2O H2O2 + 2H+ + 2e- → 2H2O H2O2 + 2H+ + 2e- → 2H2O
- 25. CI2 + SO2 + 2H2O → 2CI- + SO4 2- + 4H+ ConstructingHalf and complete redox equations (0) (-1)CI2 red - ON ↓ (+4) SO2 oxi – ON ↑ (+6) CI2 + SO2 + 2H2O→ 2CI- + SO4 2- + 4H+ Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation Oxidizing Agent Reduction CI2 + 2e → 2CI- Reducing Agent Oxidation SO2 → SO4 2- + 2e- Loss electron Increase ON ↑ Gain electron Decrease ON ↓ Completefull eqn Oxidation half eqnReduction half eqn 1. Balance # O - add H2O 2. Balance# H add H+ 3. Balance# charges - add electrons 4. Balance# electron transfer SO2 → SO4 2- x 1x 1 CI2 + 2e- → 2CI- SO2 + 2H2O → SO4 2- + 4H+ + 2e- + SO2 + 2H2O → SO4 2- SO2 + 2H2O → SO4 2- + 4H+ SO2 + 2H2O → SO4 2- + 4H+ + 2e- CI2 + SO2 + 2H2O→ 2CI- + SO4 2- + 4H+ CI2 → 2CI- CI2 + 2e- → 2CI- CI2 + 2e- → 2CI- SO2 + 2H2O → SO4 2- + 4H+ + 2e-
- 26. MnO4 - (Acidic medium) - Strong oxidizing agent - Gain 5 e- MnO4 - - (Neutral medium) - Moderate oxidizing agent - Gain 3 e MnO4 - + 2H2O + 3e- →MnO2 + 4OH- MnO4 - - (Basic medium) - Weak oxidizing agent - Gain 1 e DisproportionalReaction Substance both oxidized and reduced simultaneously Substance acts as oxidizing and reducing agent Redox Reaction (-1) Br - oxi – ON ↑ (0) (0) CI red – ON ↓ (-1) CI2 + 2KBr-→ 2KCI + Br2 Reducing agent - oxidized Oxidizingagent – reduced Oxidizing Agent Reducing Agent Concept Map Redox Reaction in diff medium (-1) H2O2 red – ON ↓ (-2) H2O2 → H2O + 1/2O2 (-1) H2O2 oxi – ON ↑ (0) (0) CI2 red – ON ↓ (-1) CI2 + H2O → HOCI + HCI (0) CI2 oxi – ON ↑ (+1) (+3) HNO2 red – ON ↓ (+2) HNO2 → HNO3 + 2NO + 2H2O (+3) HNO2 oxi – ON ↑ (+5) Cu2SO4 → CuSO4 + Cu (+1) Cu red – ON ↓ (0) (+1) Cu oxi – ON ↑ (+2) MnO4 - + 8H+ + 5e- → Mn2+ + 4H2O (+7) ON decrease ↓ (+2) (+7) ON decrease ↓ (+4) MnO4 - + e- → MnO4 2- (+7) ON decrease ↓ (+6)
- 27. Sn2+ + 2Fe3+ → Sn4+ + 2Fe2+2Fe2+ + CI2 → 2Fe3+ + 2CI-Ca + 2H+ → Ca2+ + H2 IB Redox Questions Deduce half eqn of oxidation and reduction for the following Ca + 2H+ → Ca2+ + H2 2Fe2+ + CI2 → 2Fe3+ + 2CI- Sn2+ + 2Fe3+ → Sn4+ + 2Fe2+ 0 +1 +2 0 Ca → Ca2+ + 2e 2H+ + 2e → H2 oxidation reduction +2 0 +3 -1 2Fe2+ → Fe3+ + 2e CI2 + 2e → 2CI- oxidation reduction +2 +3 +4 +2 Sn2+ → Sn4+ + 2e 2Fe3+ + 2e → 2Fe2+ Substancesacting as oxidizingand reducing agent 2MnO4 - + 5H2O2 + 6H+ → 2Mn2+ + 5O2 + 8H2O H2O2 + 2Fe2+ + 2H+ → 2Fe3+ + 2H2O H2O2 + 2I- + 2H+ → I2 + 2H2O Oxidizing Agent Reducing Agent MnO4 - Fe2+ Cr2O7 2- SO2 HNO3 I- H2O2 H2S CI2 SO3 2- Acidified H2O2 act as oxidizing agent - Oxidizes Fe2+ to Fe3+ - Oxidizes I- to I2 Acidified MnO4 - act as more powerful oxidizing agent - Oxidizes weaker oxidizing agent H2O2 to H2O and O2 - H2O2 act as reducing agent Identify oxidizingand reducing agentfor following rxn. 5As2O3 + 2MnO4 - + 16H+ → 2Mn2+ + 5As2O5 + 8H2O 2NO3 - + 3Cu + 8H+ → 3Cu2+ + 2NO+ 4H2O Cr2O7 2- + 3NO2 - + 8H+ → 2Cr3+ + 3NO3 - + 4H2O 1 2 3 oxidizing agent oxidizing agent oxidizing agent reducing agent reducing agent reducing agent
