T16 IB Chemistry Chemical Kinetics
- 1. T6/16- Chemical kinetics Picture and some content from AS boardworks ©
- 2. Lesson outline…. • Lesson 1- Topic 6 review. • Lesson 2- All about the rate equation. • Lesson 3- Determining a rate equation experimentally(P). • Lesson 4- Evaluation of the iodination of propanone. • Lesson 5- Evaluating proposed reaction mechanisms. • Lesson 6- The Arrhenius equation. • Lesson 7- Determining Ea experimentally (P). • Lesson 8- exam question review☺ *Note: P= Practical☺
- 3. 16.1- Rate expression and reaction mechanism
- 4. Lesson 1- Topic 6 review. Level 4: State the main principles of collision theory. Level 7: Explain how an increase in temperature affects the activation energy (Ea)of a chemical reaction. Level 5/6: Describe at least two ways of determining the rate of a chemical reaction.
- 5. Topic 6 review questions- see Pearson questions (MCQ and short answer)
- 6. Starter
- 10. Homework task…. • Introduction to ‘kognity’ • Complete the SL review task on topic 6: https://kristin.kognity.com/schoolstaff/app/che mistry-hl-2016/assignment/44231/
- 11. Lesson 2- All about the rate equation. Level 4: Label all aspects of a rate equation/expression. Level 7: Explain the difference between a zero, first and second order reaction using graphs, text and numbers. Level 5/6: Deduce the overall order of a reaction from given information.
- 12. The rate equation/expression The rate equation is an equation that relates the concentrations of substances involved in a reaction to the rate of the reaction. For the reaction A + B C the rate of reaction depends on the concentrations of A and B ([A] and [B]) and various constants in the following way: Rate = k[A]m [B]n k is the rate constant (units depend of values of m and n) m is the order of reaction with respect to A n is the order of reaction with respect to B. m + n = overall order of the reaction
- 13. Determining the rate equation The rate equation can be determined by completing a series of experiments varying the concentrations of each of the reactants. A similar set of experiments can be carried out keeping [A] constant and varying [B] to determine how changing [B] affects the rate of reaction. To determine how [A] affects the rate, several different experiments can be carried out in which [B] is kept constant and [A] is changed. The data can then be used to work out the relationship between rate and [A]. A + B C *See iodine and propanone investigation in lesson 3.
- 15. Two very useful graphs to remember… Taken from Pearson 2nd edition page 293
- 16. The effect of temperature on k When temperature increases, rate of reaction increases. This is because the rate constant, k, increases with temperature. 556 575 629 666 700 781 4.45 × 10–5 1.37 × 10–4 2.52 × 10–3 1.41 × 10–2 6.43 × 10–2 1.34 temp. (K) k(moldm3s–1) temperature (K) rate constant, k (moldm3 s–1) Note: the rate constant k is written as a lower case letter to distinguish it from K for kelvin or Kc for equilibrium constant.
- 17. Check for learning…. See homework questions from Pearson on reaction order and the rate equation.
- 18. Lesson 3- Determining the rate expression experimentally (P). Level 4: Measure the rate of a reaction in response to changing [reactant]. Level 7: Explain why measuring time alone is not an indication of the rate of a reaction. Level 5/6: Calculate the values of x, y and z (reactant orders) and determine the rate expression.
- 19. starter
- 20. Aim: to determine the overall order of a reaction experimentally. • See method from inthinking- I2 + propanone. Mixture [CH3COCH3] mmol.dm-3 [I2]/ mmol.dm-3 [H+]/ mmol.dm-3 Time for colour change (s) A 400 1.00 400 B 800 1.00 400 C 1200 1.00 400 D 400 0.50 400 E 400 0.25 400 F 400 1.00 800 G 400 1.00 1200 *The points where the concentration of a reactant has been changed are highlighted in green.
- 21. Lesson 4- Evaluation of the iodination of propanone ( ‘acetone’). Level 4: State the overall order of this chemical reaction. Level 7: Evaluate some of the assumptions which have been made in this practical. Level 5/6: Calculate the rate constant for this reaction based on your experiment.
- 22. starter The literature value for the rate constant of this particular reaction is given as: K= 3.76 x 10-5 How does your rate constant compare to this value?
- 23. Interpreting your results…. Your experimental data may be used to answer the following questions: 1. Calculate the order with respect to propanone. 2. Calculate the order with respect to Iodine. 3. Calculate the order with respect to H+. 4. Deduce the rate equation for this reaction. 5. *Calculate a value for the rate constant (k) 6. Derive the units of the rate constant.
- 24. Aim: to determine the overall order of a reaction experimentally. • See method from inthinking- I2 + propanone. [CH3COCH3] mmol.dm-3 [I2]/ mmol.dm-3 [H+]/ mmol.dm-3 Time for colour change (s) Rate= ∆ [I2]/time (mmol.dm-3.s-1) Rate constant k (units?) a. 400 1.00 400 49.42 0.020 b. 800 1.00 400 24.58 0.041 c. 1200 1.00 400 17.94 0.056 d. 400 0.50 400 26.11 0.019 e. 400 0.25 400 15.36 0.016 f. 400 1.00 800 27.54 0.036 g. 400 1.00 1200 15.48 0.065 *Note: we’re assuming here that all of the I2 is used up and so the change in concentration is initial- 0 ☺ e.g. for mixture a, it would be 1.00- 0.00 = 1.00 mol.dm-3
- 25. Assumptions made in this experiment…. • we’re assuming here that all of the I2 is used up and so the change in concentration is initial- 0 ☺ e.g. for mixture a, it would be 1.00- 0.00 = 1.00 mol.dm-3 • Assuming that we are detecting the colour change at the right time. • Assume that the temperature is constant (remember the value of k is affected by temperature)
- 26. Lesson 5- Evaluating proposed reaction mechanisms. Level 4: Define what the rate determining step of a reaction mechanism is. Level 7: Explain why a reactant which has a zero order effect on the rate does not appear in the rate expression. Level 5/6: Deduce the rate expression of a reaction based on a proposed reaction mechanism.
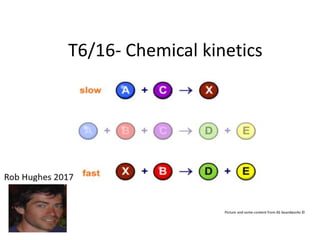
- 27. More about the rate determining step
- 28. The rate equation and mechanisms
- 29. Potential energy profile for a two step reaction (from Pearson HL pg 297)
- 30. What do exam questions look like? (taken from Pearson 2nd ed HL pg 300)
- 31. A question with a twist! (Pearson page 299) Q. Determine the rate expression and thus, the overall reaction order. Q. Determine the rate expression and the overall rate of this reaction. Initially it looks like rate= k [N2O2] [O2] But, because the production of the N2O2 is reliant on the reaction between the two moleucules of NO, the [N2O2] is replaced by [NO]2 in the expression to give: Rate = k [NO]2[O2] with an overall reaction order of 3 Not 2! * Remember- golden rule, if a species appears before or in the rate determining step then it must be accounted for in the rate expression!
- 32. More practice! 1. Questions on kognity ☺
- 34. Lesson 6- The Arrhenius equation. Level 4: Recall that the rate constant, k, is temperature dependent. Level 7: Calculate the activation energy of a reaction from values of the rate constant at only 2 temperatures. Level 5/6: Calculate the activation energy (Ea) of a reaction over a range of temperatures using the Arrhenius equation.
- 36. The Arrhenius Equation •Collision Theory: A bimolecular reaction occurs when two correctly oriented molecules collide with sufficient energy. •Activation Energy (Ea): The potential energy barrier that must be surmounted before reactants can be converted to products.
- 37. 1. Arrhenius considered the activation energy of a reaction (Ea)
- 38. 2. Arrhenius considered the frequency of collisions with correct orientation (A) (‘the frequency factor’)
- 39. 3. Arrhenius considered the fact that temperature affects the rate constant of a reaction, where k is proportional to temperature (T) 556 575 629 666 700 781 4.45 × 10–5 1.37 × 10–4 2.52 × 10–3 1.41 × 10–2 6.43 × 10–2 1.34 temp. (K) k(moldm3s–1) temperature (K) rate constant, k (moldm3 s–1) Note: the rate constant k is written as a lower case letter (in italic) to distinguish it from K for kelvin or Kc for equilibrium constant.
- 40. The Arrhenius Equation (*see section 1 of d.book) •This relationship is summarized by the Arrhenius equation. •Taking natural logs and rearranging, we get: lnk = - Ea R 1 T + lnA k = Ae - Ea RT
- 41. 1. Using the Arrhenius Equation Temp (1/T)K-1 k (M-1 s-1) 0.0018 3.52e-7 0.0016 3.02e-5 0.0015 2.19e-4 0.0014 1.16e-3 0.0012 3.95e-2 * remember- convert temperature readings to Kelvin (add 273)
- 42. Using the Arrhenius Equation (graphing method) The second-order rate constant for the decomposition of nitrous oxide (N2O) into nitrogen molecule and oxygen atom has been measured at different temperatures: Determine graphically the activation energy for the reaction. k (M -1 s-1 ) t (°C ) 1.87x10-3 600 0.0113 650 0.0569 700 0.244 750 The second-order rate constant for the decomposition of nitrous oxide (N2O) into nitrogen and oxygen has been measured at different temperatures: Determine graphically the activation energy for the reaction. Note: use logger pro☺ K mol.dm-3.s-1 t (°C ) 1.87x10-3 600 0.0113 650 0.0569 700 0.244 750
- 43. 2. Using the Arrhenius Equation (simultaneous equations method ‘2-point method’) see section 1 of d.book -= 122 1 11 ln TTR E k k a Pearson pg 303
- 44. The answer! Ensure you can carry out this calculation on your calculator!
- 45. Check for learning…. (taken from Pearson page 304)
- 46. Lesson 7- Determining Ea experimentally (P). Level 4: Recall that the slope of an Arrhenius plot is equal to Ea/R. Level 7: Determine the experimental error in your calculated Ea value. Level 5/6: Determine the Ea of a reaction from experimental data.
- 47. Aim: to determine the Ea of a reaction from experiment and graphical technique. See practical from inthinking. Link to shared results doc.: https://docs.google.com/spreadsheets/d/1RhlIt- a1H3Py0yi8tM9mqxvXdN8Q1Uq_agPfVwPg0Ng/edit#gid =0
- 48. Lesson 8 Review of topic- IB exam questions☺ *See practice questions from Pearson.
- 49. Starter- if slope = -Ea/R, what was the activation energy for this reaction?
- 50. Past paper questions • See question set from Pearson (~1.5 hours work☺)