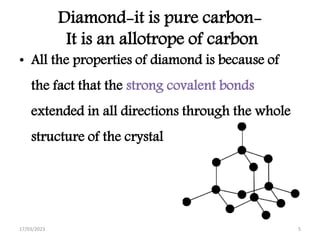
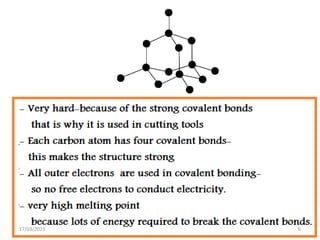
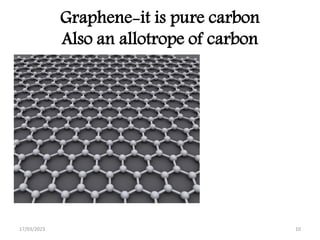
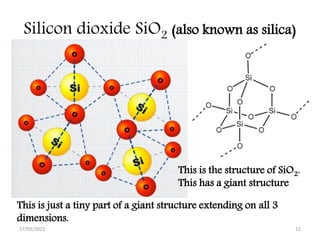
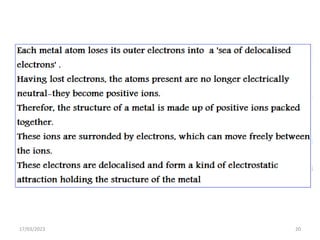
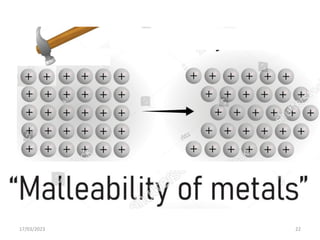
Giant covalent and ionic structures form when many atoms are bonded together in repeating patterns. Giant covalent structures include carbon materials like diamond and graphite, which have carbon atoms bonded together in tetrahedral or planar arrangements. Silicon dioxide also has a giant covalent structure where silicon atoms are bonded to four oxygen atoms in a repeating pattern. Giant ionic structures form crystalline ionic compounds where ions are bonded via electrostatic forces, such as sodium chloride which contains sodium and chloride ions. These giant structures have high melting points due to the large amount of energy required to overcome the numerous bonds between constituent atoms.