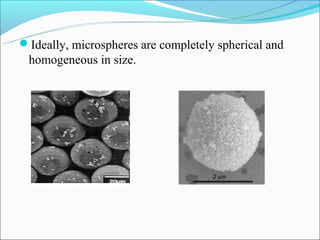
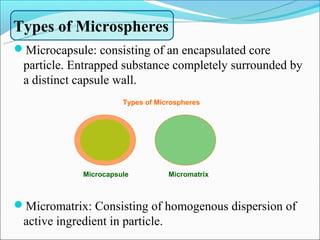
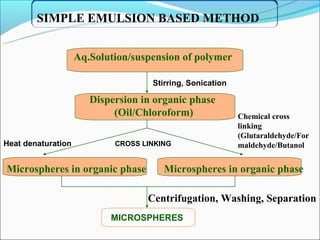
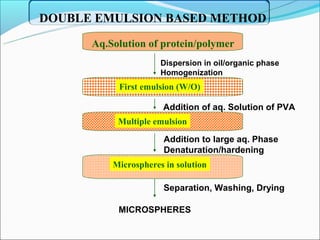
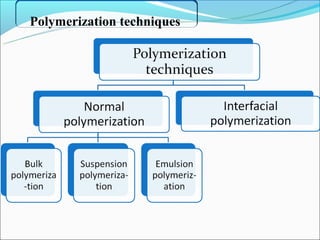
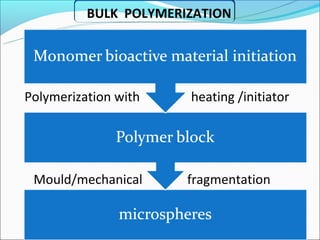
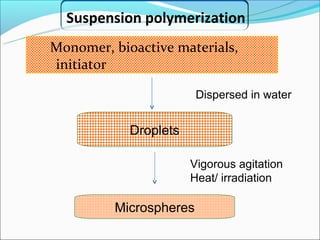
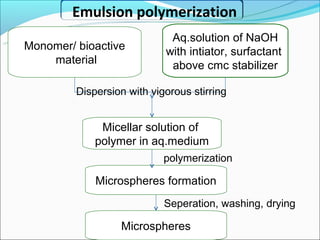
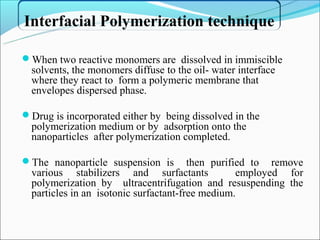
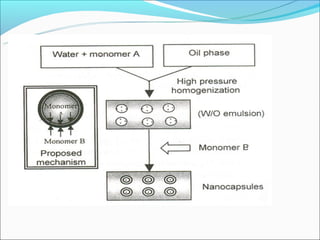
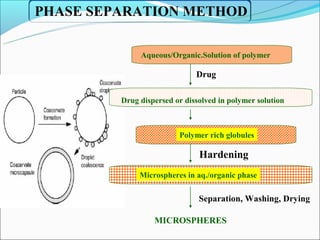
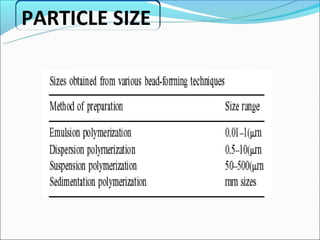
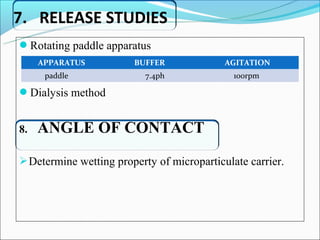
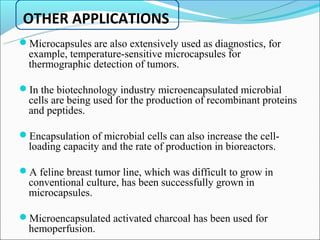
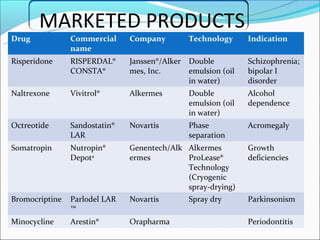
This document summarizes a seminar on microsphere drug delivery systems. It discusses the classification, preparation methods, drug release mechanisms, and characterization of microspheres. The main preparation techniques covered are single and double emulsion methods, polymerization, and phase separation. Microspheres can provide controlled release and targeting of drugs. They have applications in taste masking, converting liquids to solids, and protecting drugs. Some marketed microsphere products are listed.