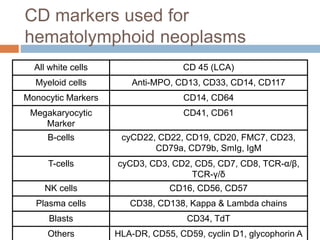
Flow cytometry allows for the quantitative and qualitative analysis of cell properties as cells flow in a fluid stream through a laser. Cells are labeled with fluorescent markers and pass through the laser one by one. Light scattering and fluorescence emission are converted to digital signals which provide information on cell size, granularity, and marker expression. Data is displayed as histograms, dot plots, or density plots to identify cell populations and phenotypes.