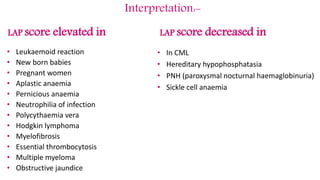
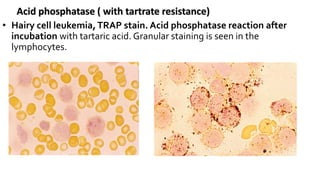
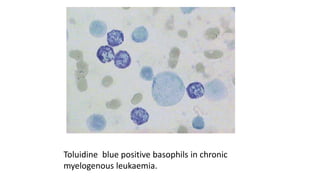
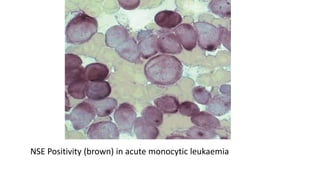
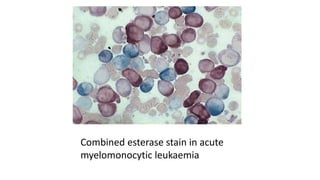
This document provides information on various cytochemical staining techniques used in hematology, including myeloperoxidase, esterase, alkaline phosphatase, acid phosphatase, Sudan black B, periodic acid Schiff, and Toluidine blue staining. It describes the principle, reagents, procedure, and interpretation for each stain. These stains are used to classify and diagnose different types of leukemia by identifying cellular enzymes and components in blood and bone marrow samples.





































![Periodic Acid – Schiff [PAS] Reaction
Giant multinucleate late normoblasts
(left). Granular PAS positivity in
proerythroblasts and homogeneous
positivity in the later normoblasts](https://image.slidesharecdn.com/cytochemicalstainingchecked-160629070928/85/Cytochemical-staining-checked-40-320.jpg)










![Perl’s Iron stain
(Prussian Blue Reaction):
• Principle:
• Sidrotic granules are found in the cytoplasm of developing cells
in [BM] in the form of Ferric [Fe+3].
• Perls' reagent is formed of (Potassium Ferricyanide + HCL)
• Sidrotic granules are found in nRBCs, some reticulocytes](https://image.slidesharecdn.com/cytochemicalstainingchecked-160629070928/85/Cytochemical-staining-checked-54-320.jpg)

