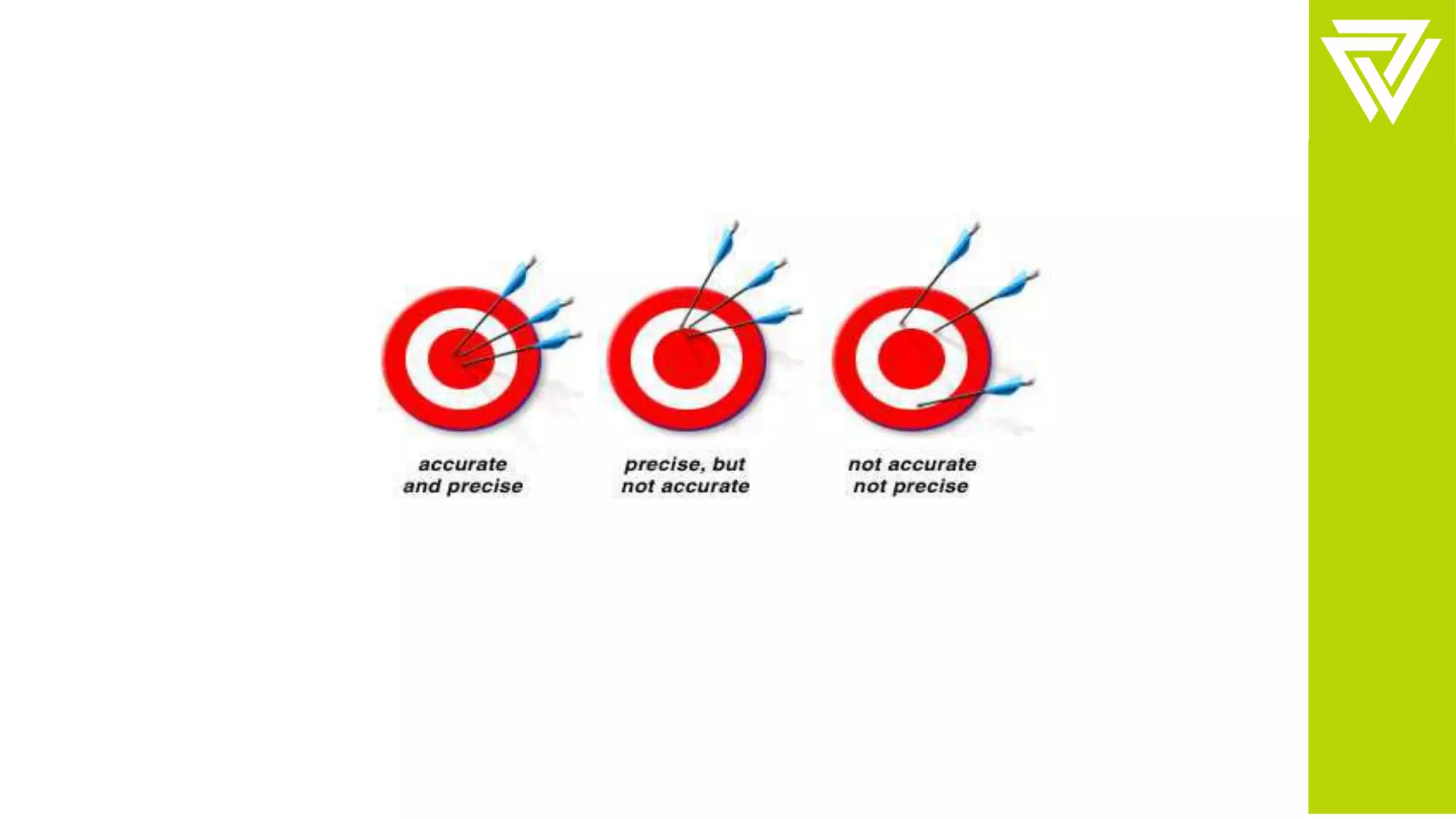
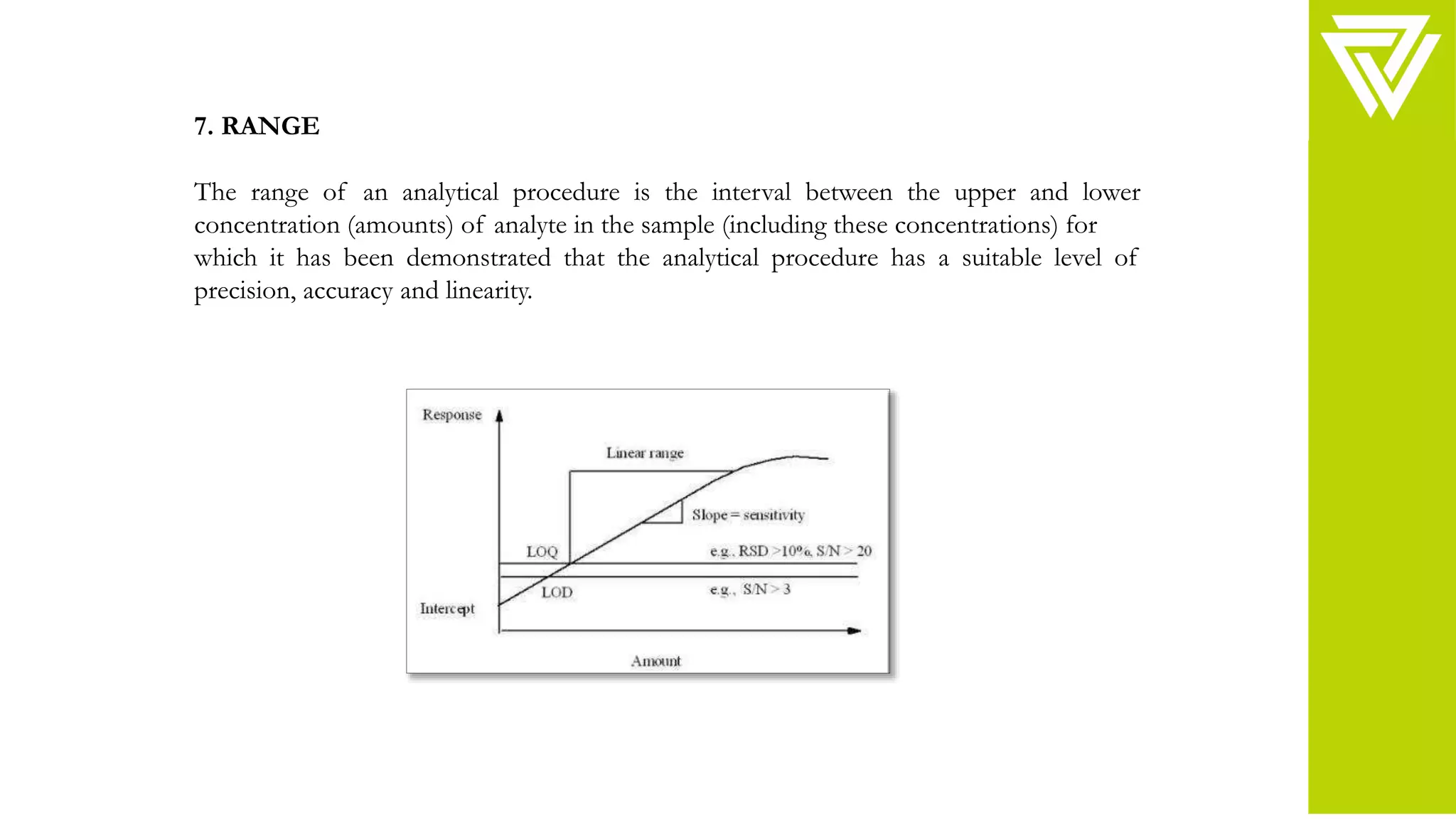
This document discusses validation of analytical procedures. It is divided into two parts. Part I provides definitions and discusses typical validation characteristics such as accuracy, precision, specificity, detection limit, and quantitation limit. Part II provides more detailed methodology guidance on how to validate these characteristics. It describes how to validate specificity, accuracy, precision, detection limit, quantitation limit, linearity, range, and robustness of analytical procedures. The goal of validation is to demonstrate that analytical procedures are suitable for their intended purpose in identifying, quantifying, and testing impurities in drug substances and products.