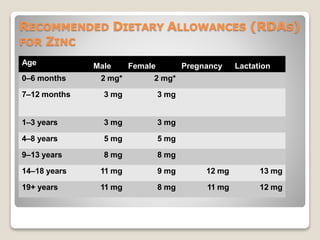
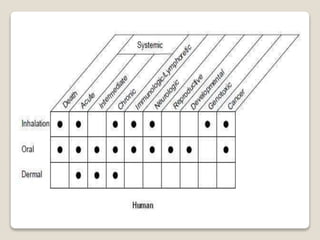
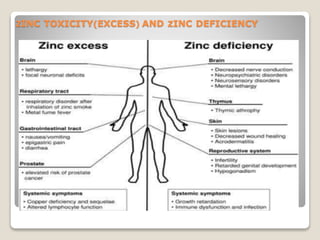
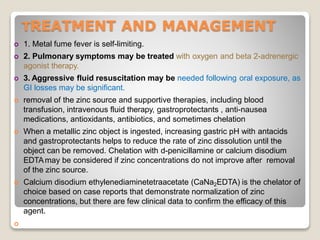
Zinc is an essential mineral that is naturally present in some foods. In small amounts, zinc is necessary for cell growth and immune function. However, too much zinc from supplements can lead to toxicity. Acute zinc toxicity may occur after ingesting zinc salts and cause nausea, vomiting, and stomach pain. Long-term high intake of zinc can deplete copper levels and impact blood lipid levels and the immune system. Occupational zinc inhalation from welding fumes can cause metal fume fever, a temporary flu-like illness. Treatment focuses on removing the zinc source, and chelation may be used for severe cases.