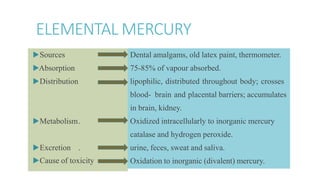
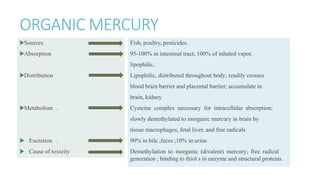
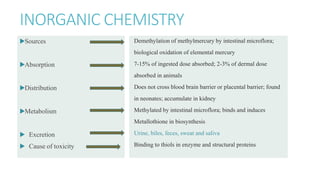
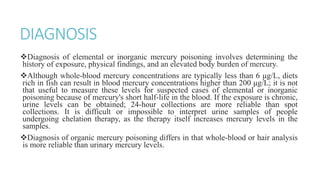
Mercury poisoning can occur through exposure to mercury in various forms from environmental and occupational sources. Symptoms vary based on the specific form but can include neurological, gastrointestinal, and renal issues. Diagnosis involves considering exposure history and measuring mercury levels in blood, urine, or hair. Chelating agents such as dimercaprol or DMSA are used for treatment to enhance mercury excretion, especially for organic mercury poisoning. Maintaining kidney function is also important for treatment.