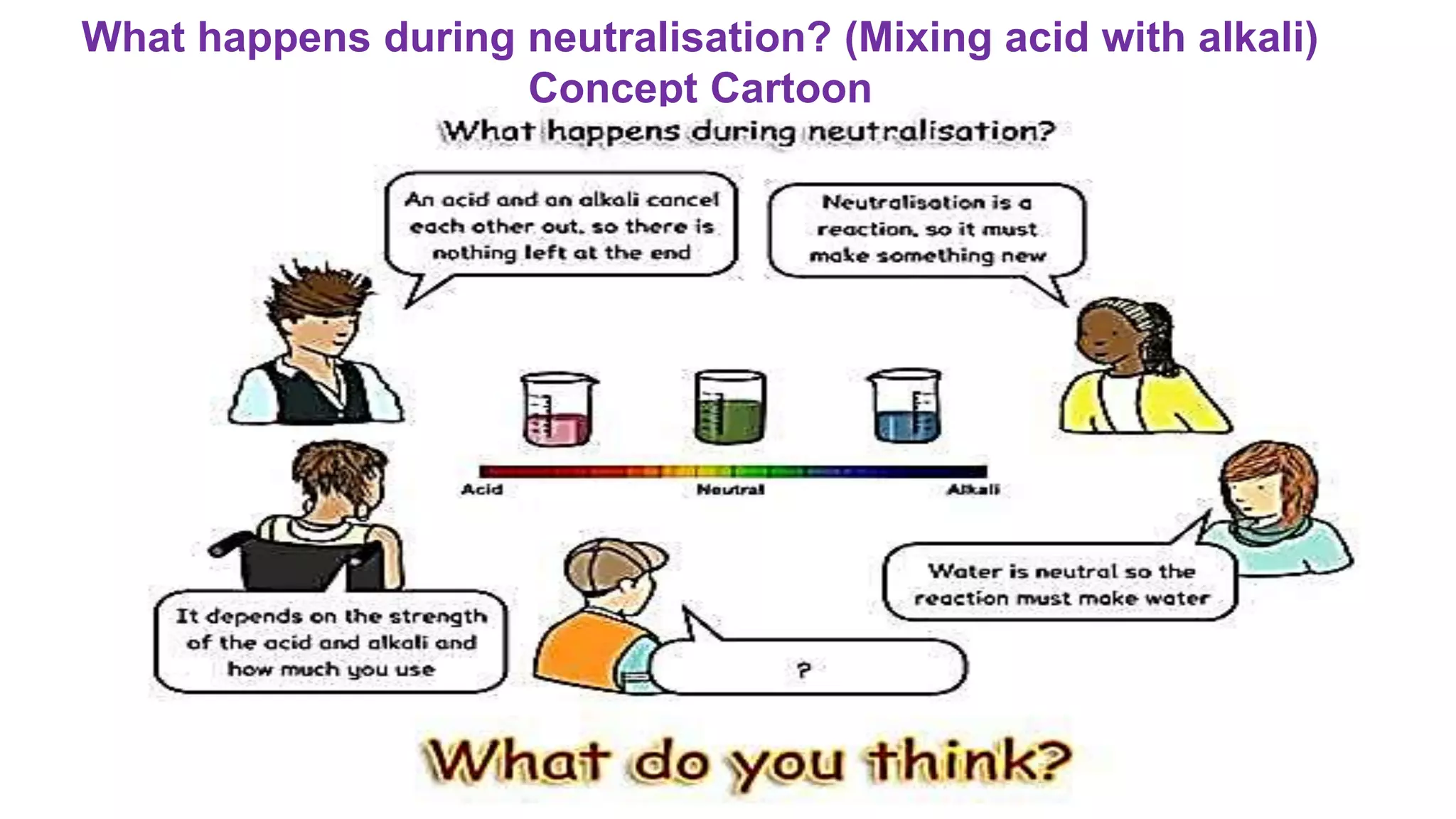
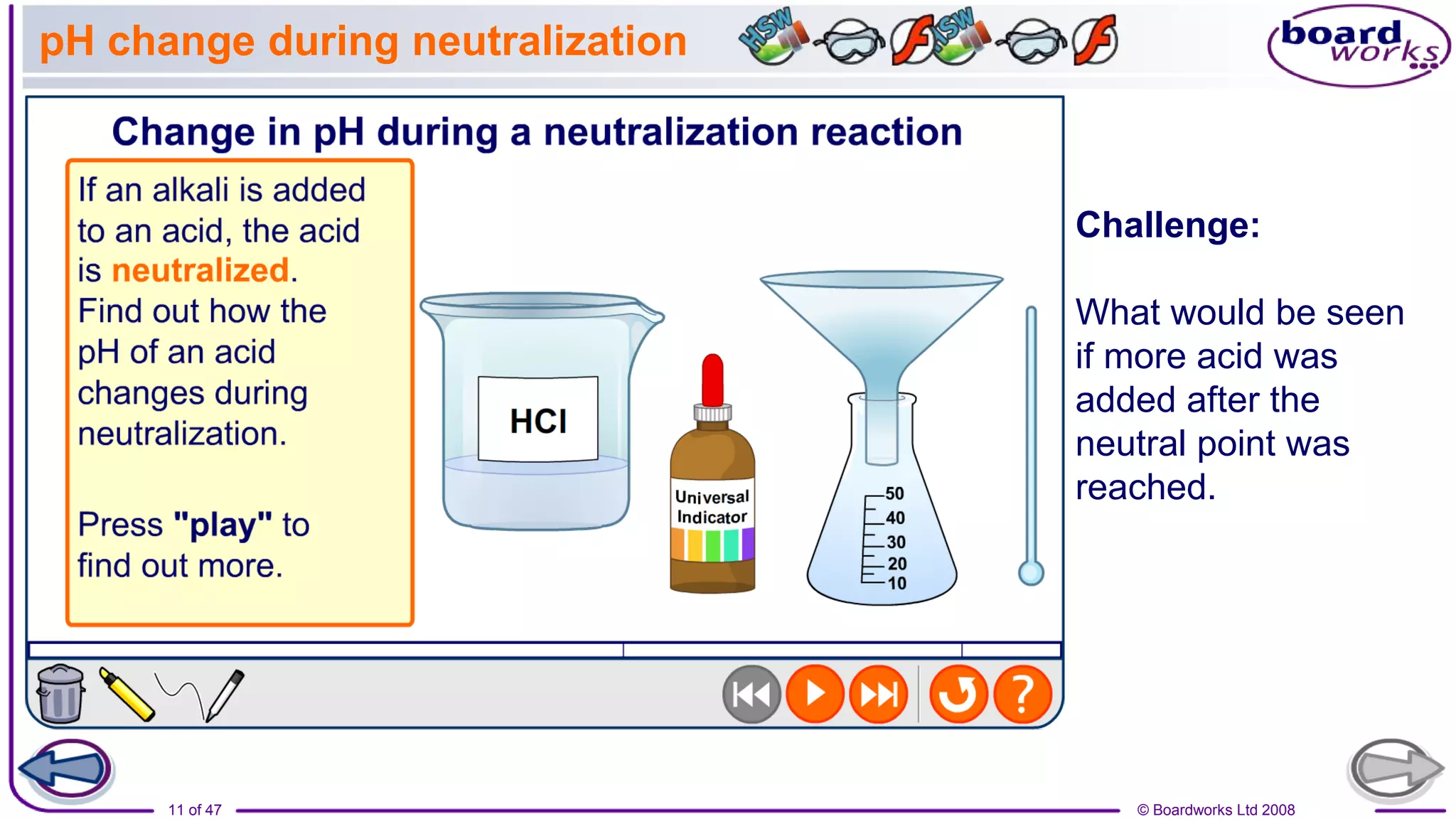
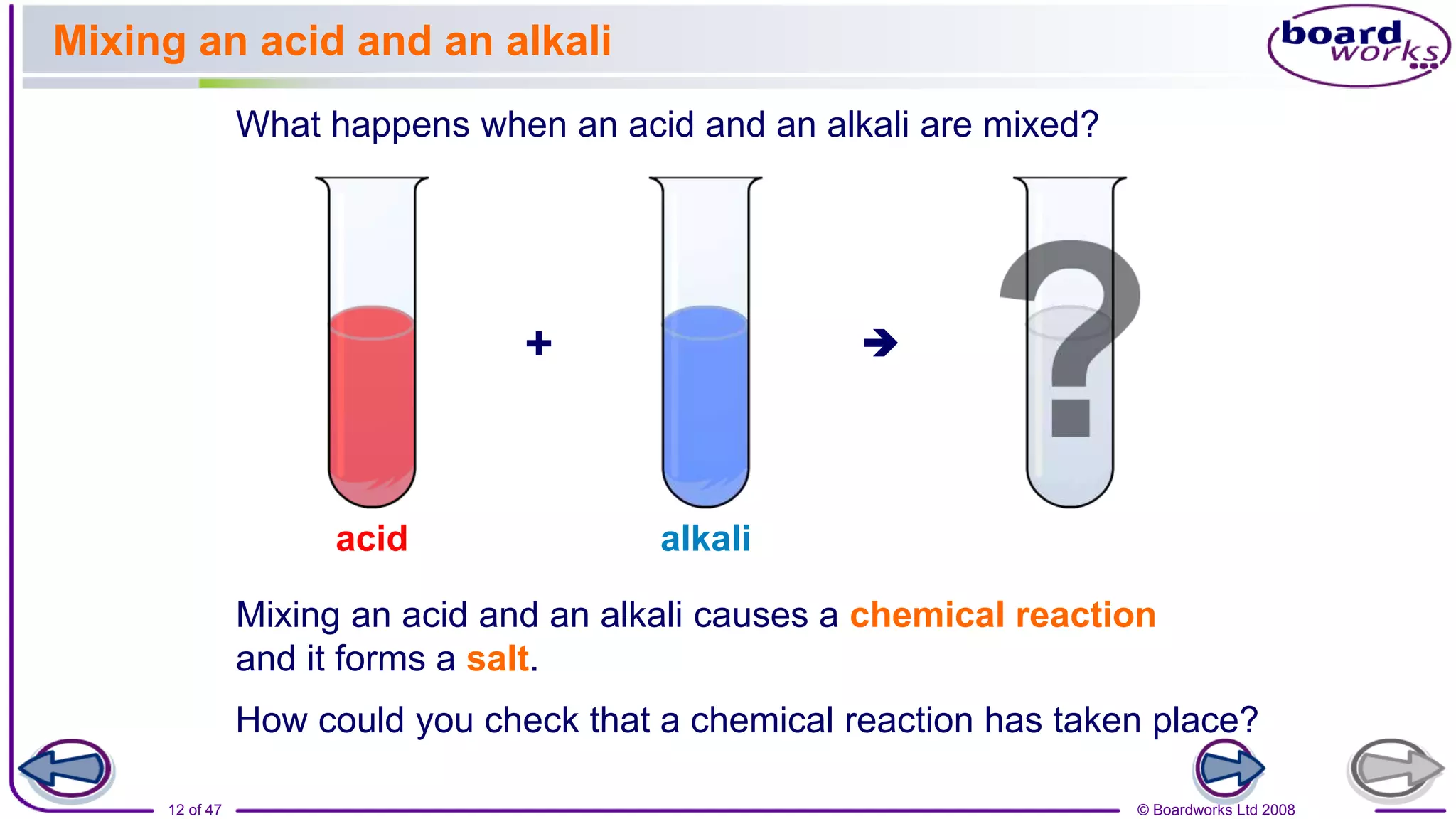
The document discusses a lesson on neutralization reactions that includes several learning activities and assessments. The key points are:
1. The lesson objectives are to describe examples of neutralization reactions and explain how pH changes during neutralization reactions using indicators.
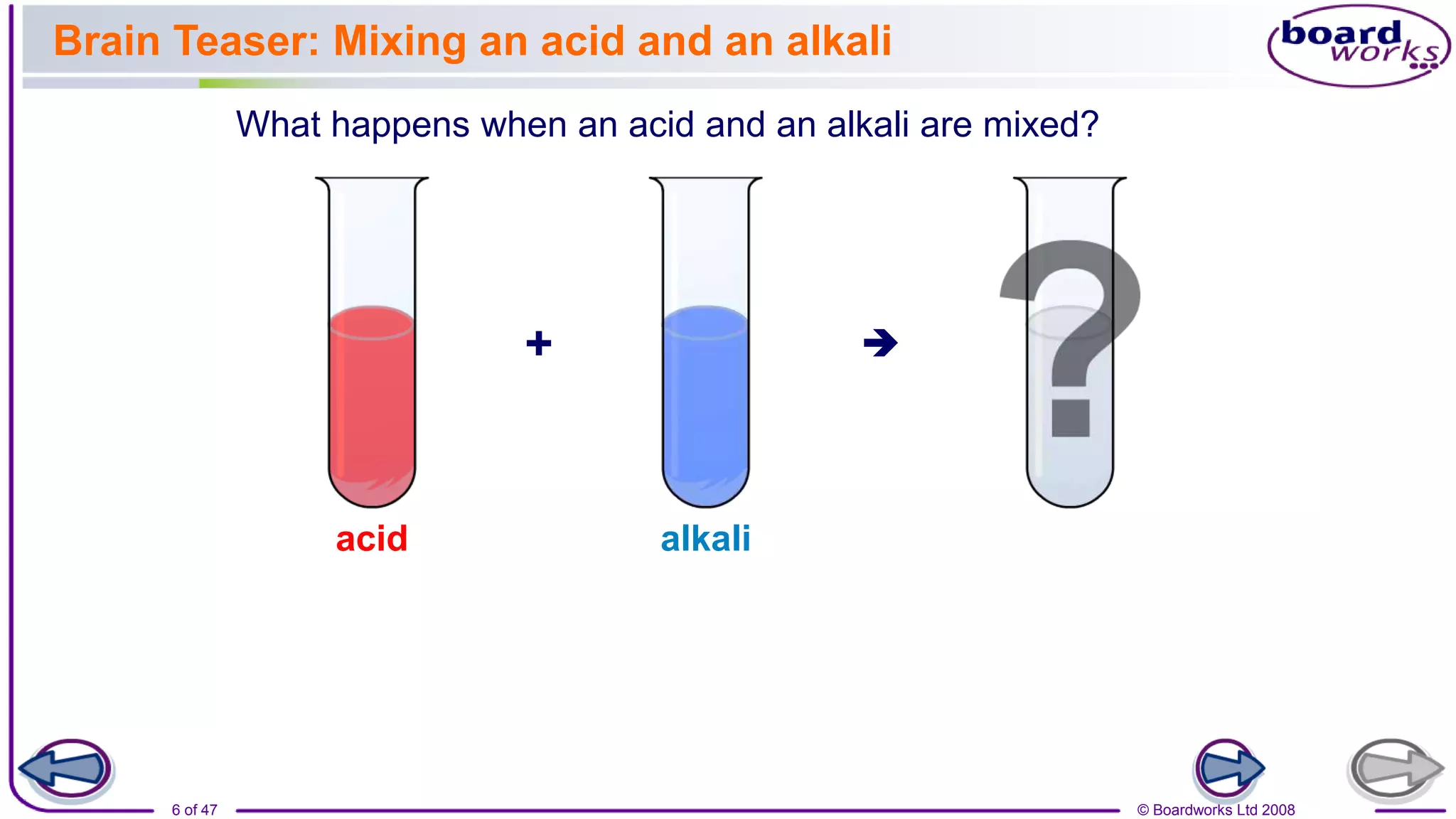
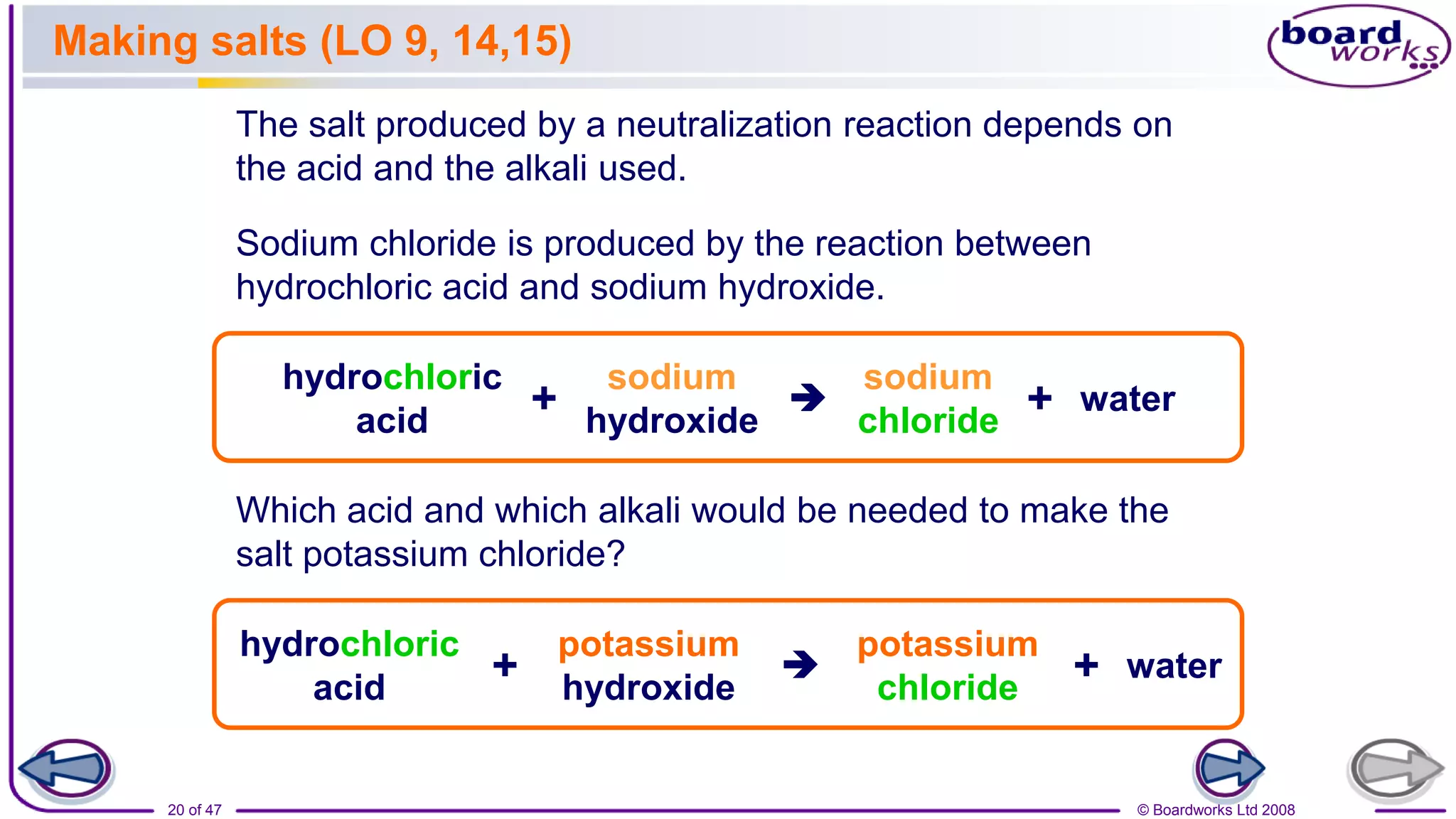
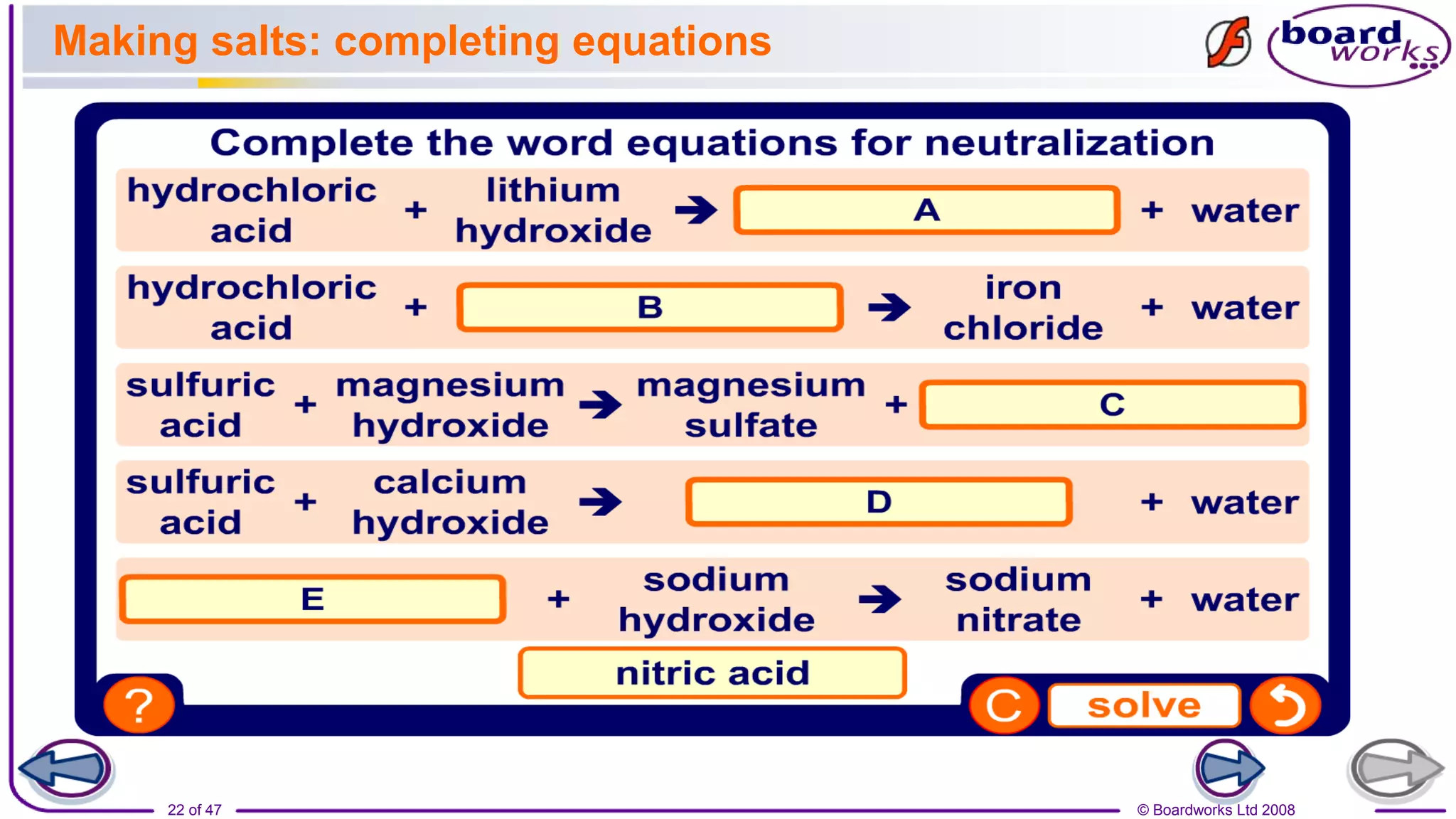
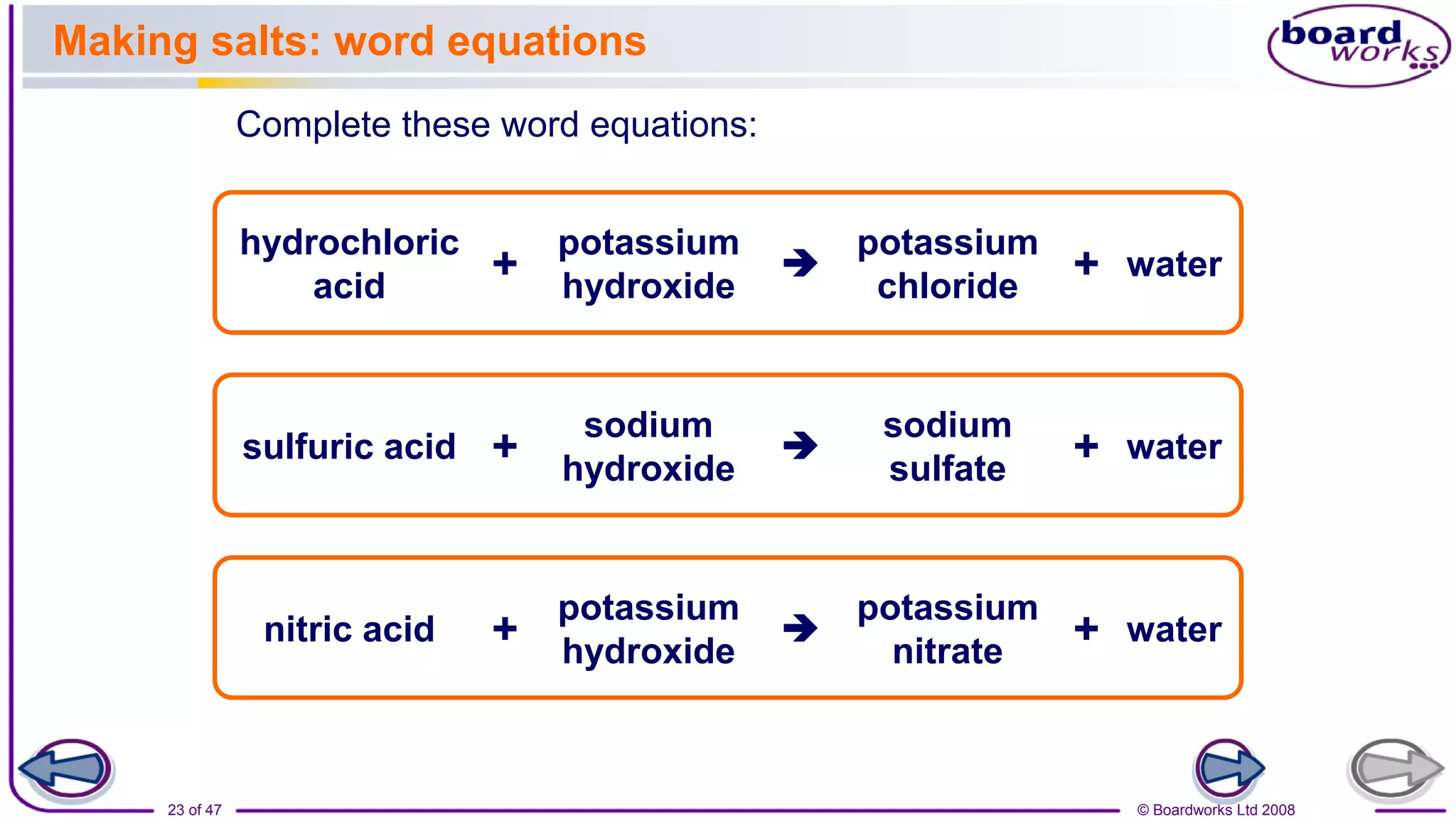
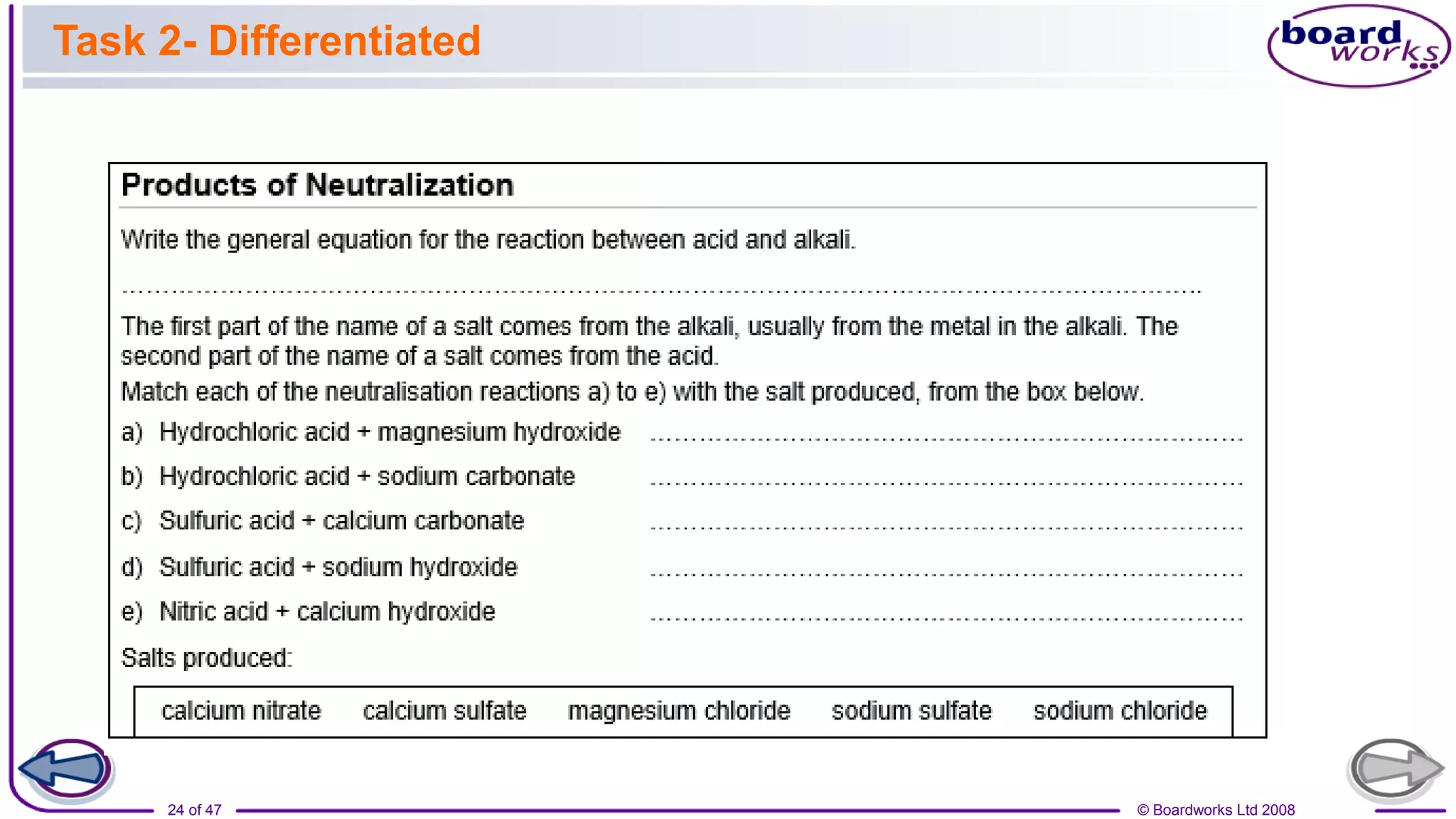
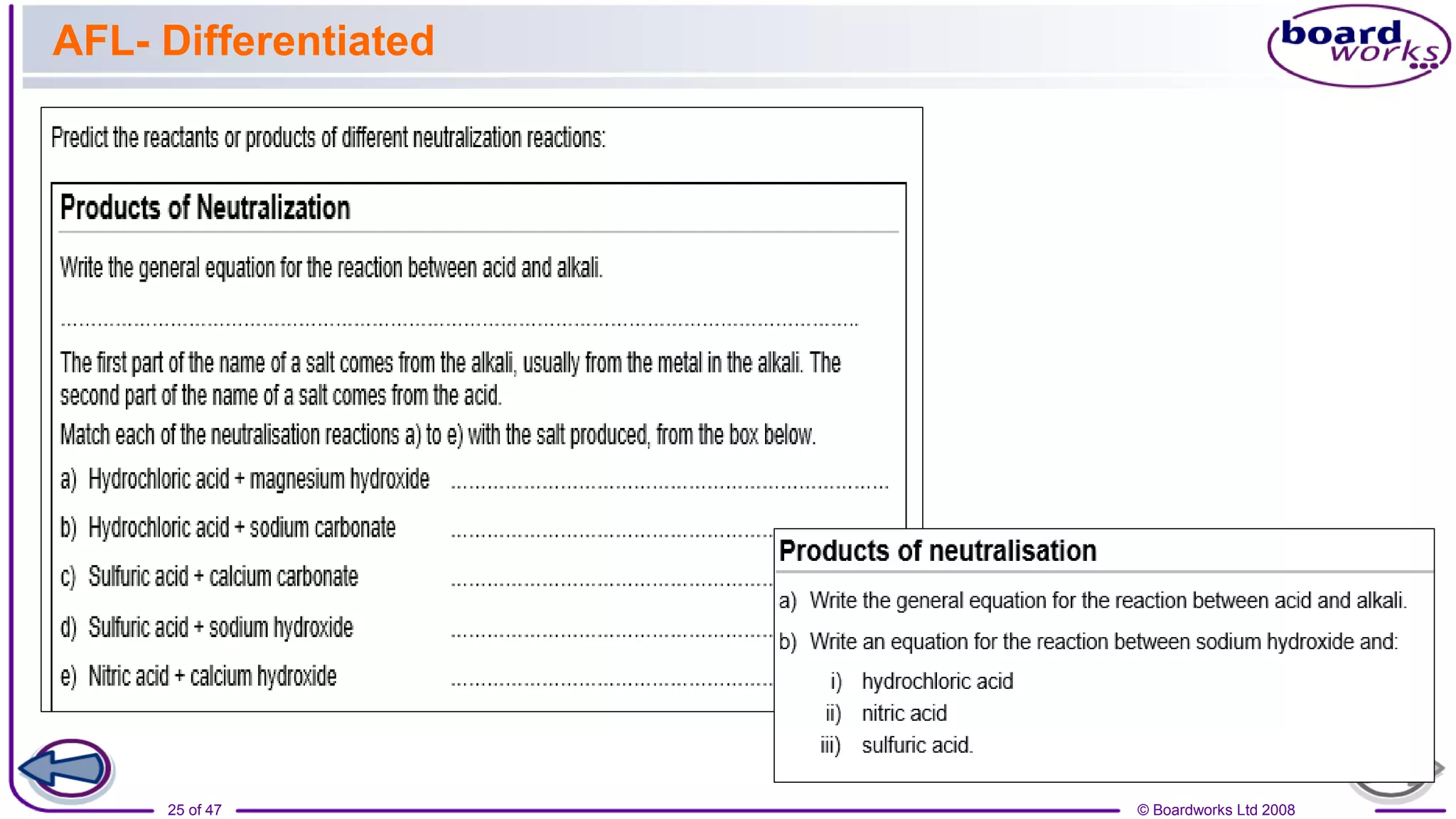
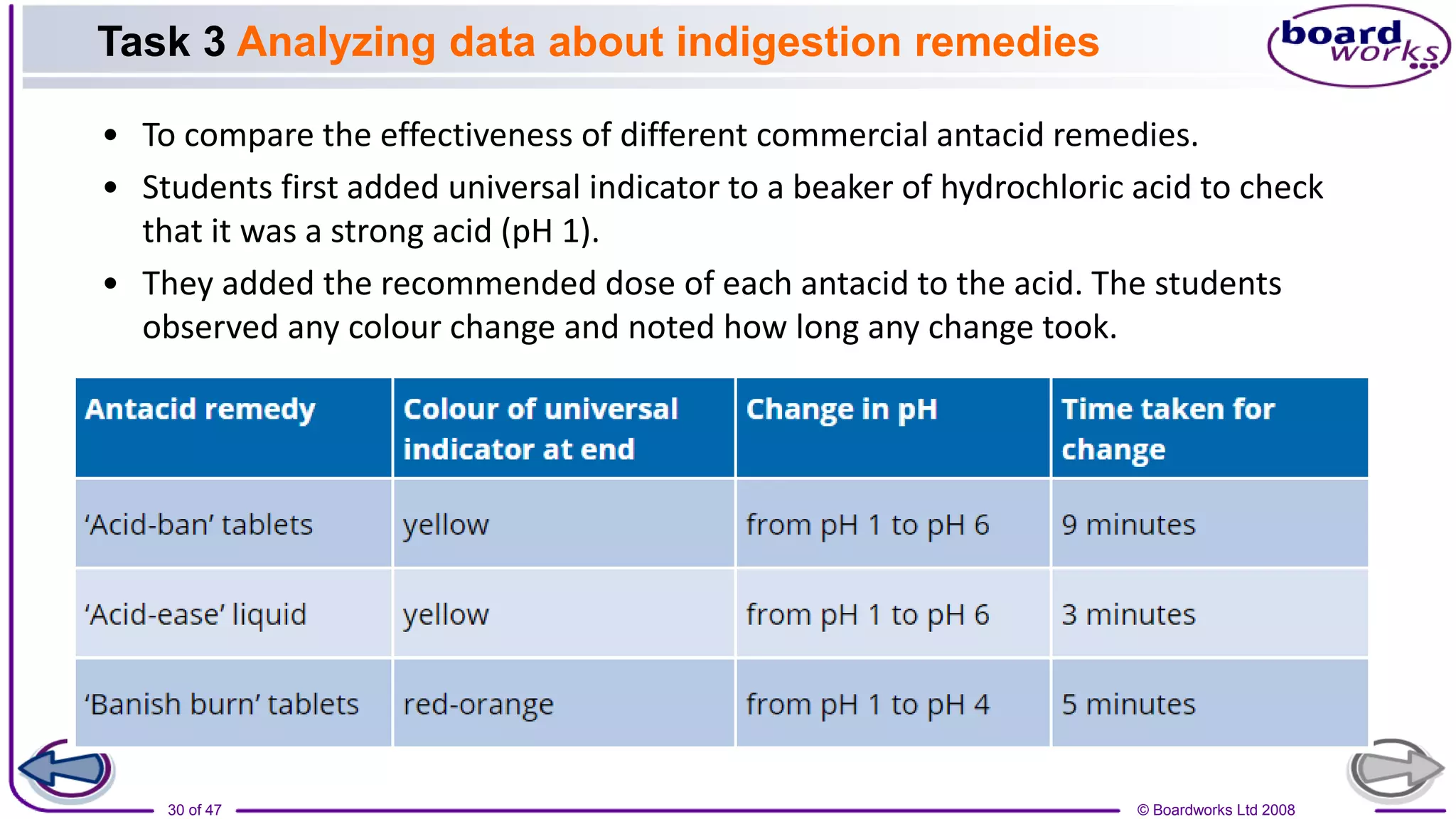
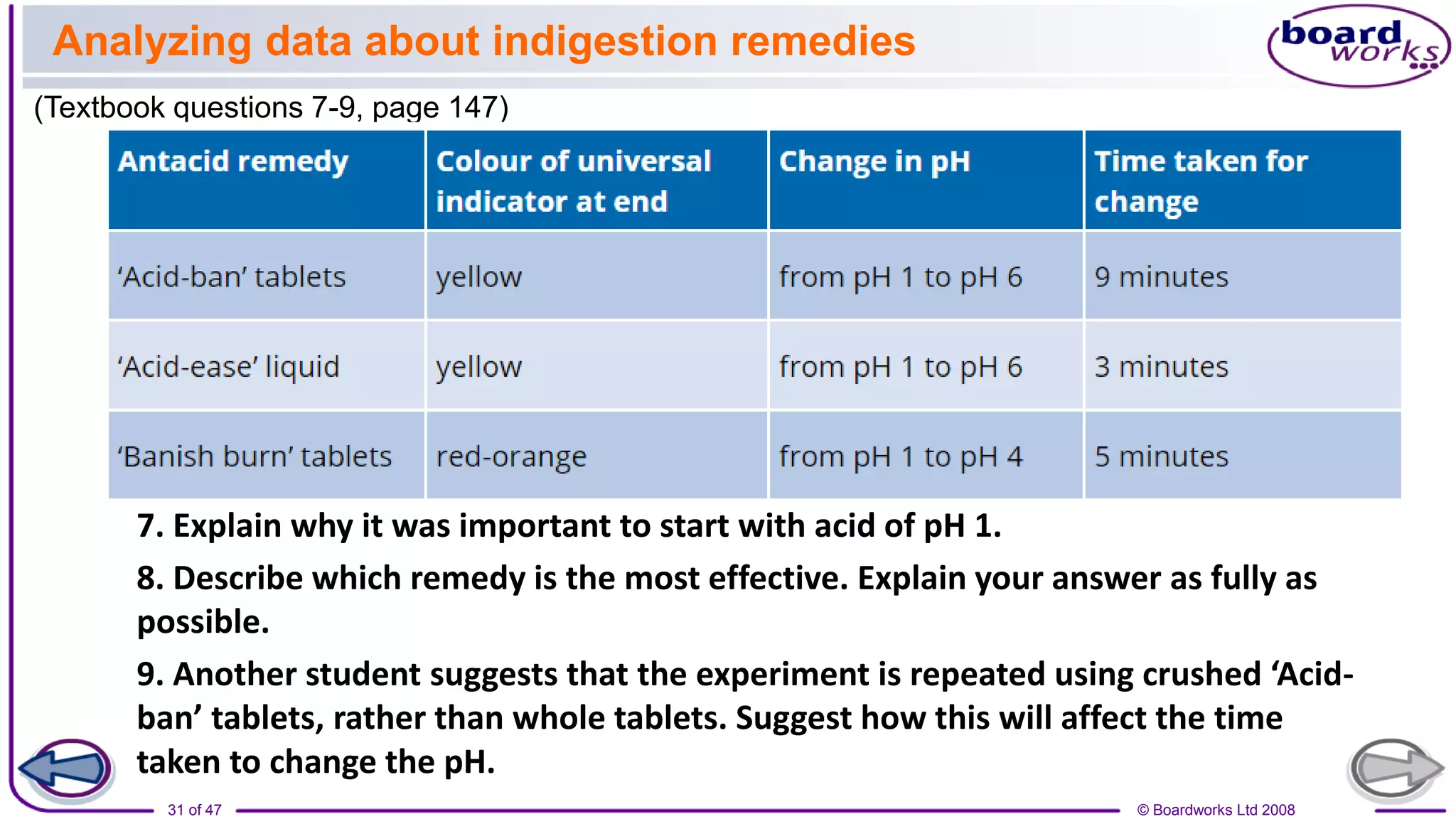
2. Learning activities include predicting reactants and products of neutralization reactions, explaining the formation of salts and water, and analyzing data to determine the most effective indigestion remedy.
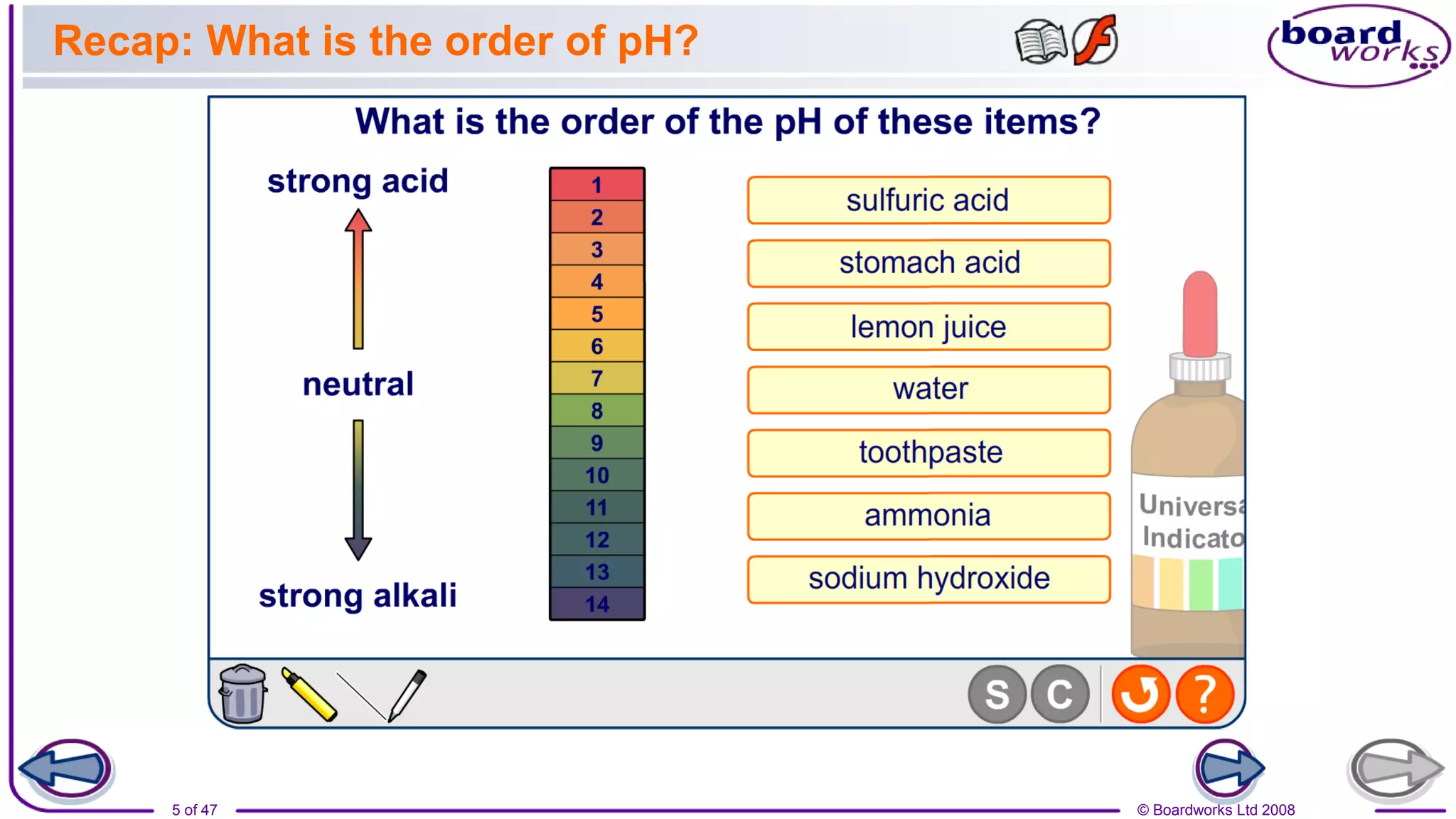
3. Assessments are used to check students' understanding of neutralization concepts like pH changes and examples of common neutralization reactions and their products.