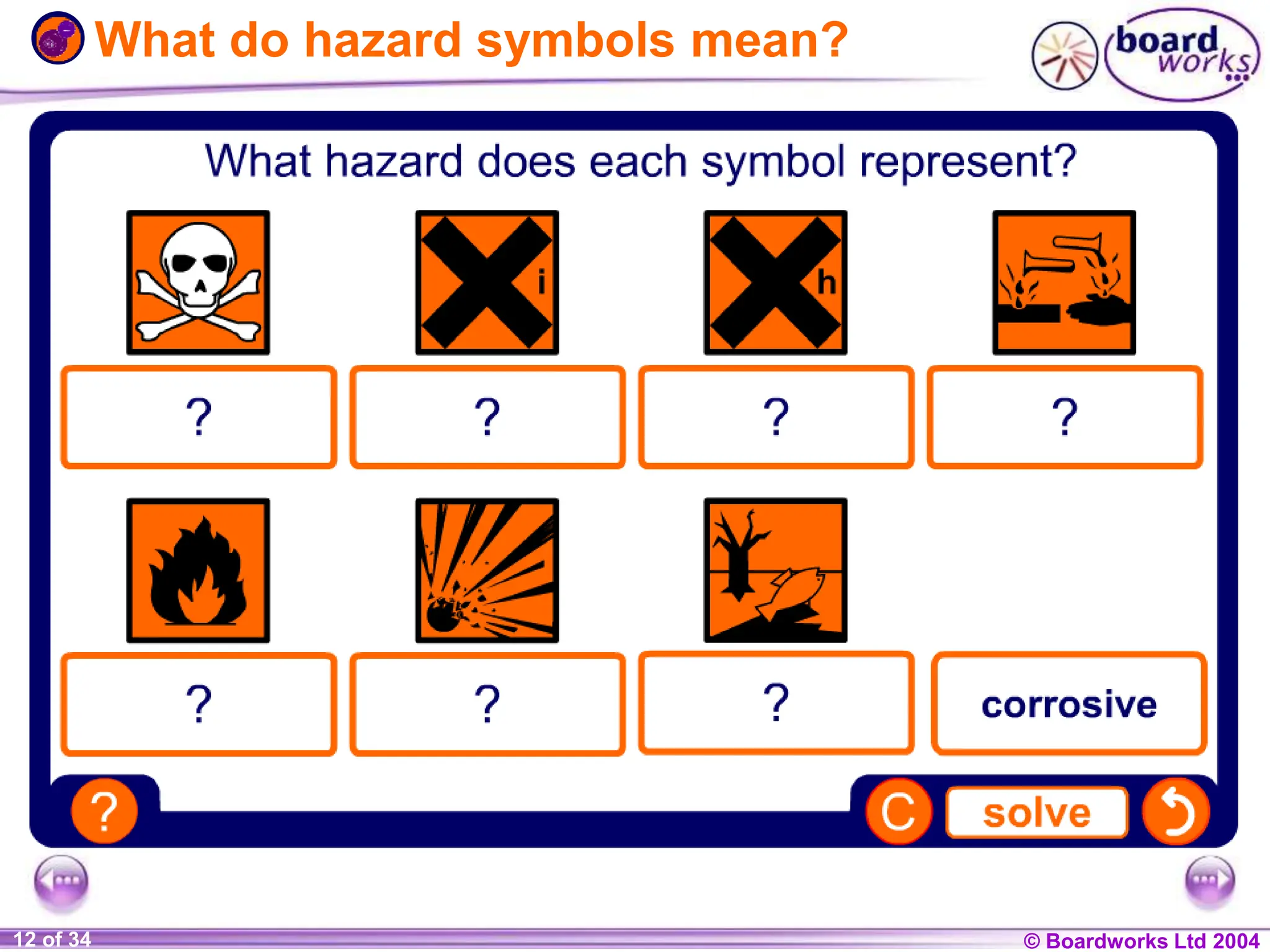
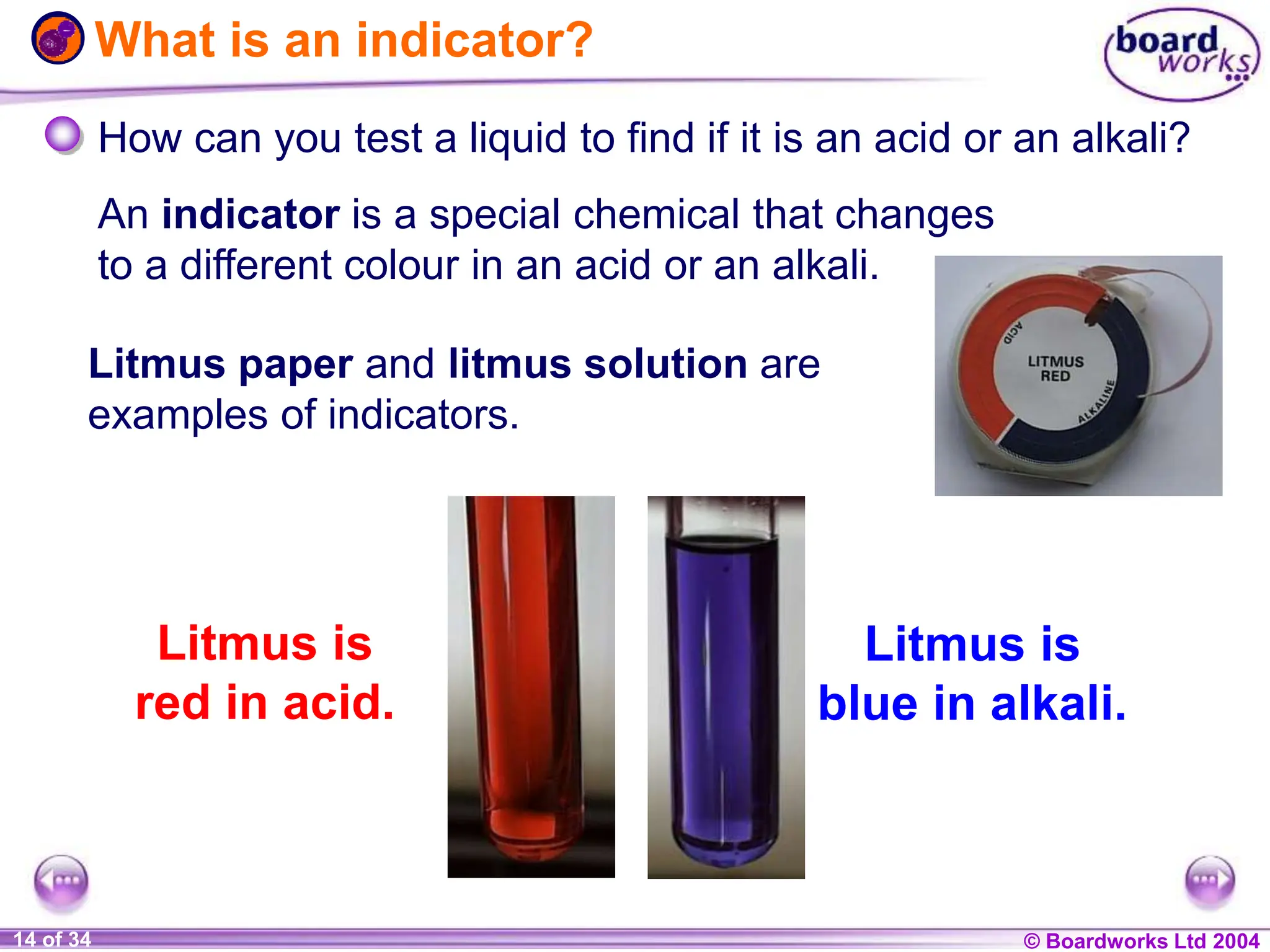
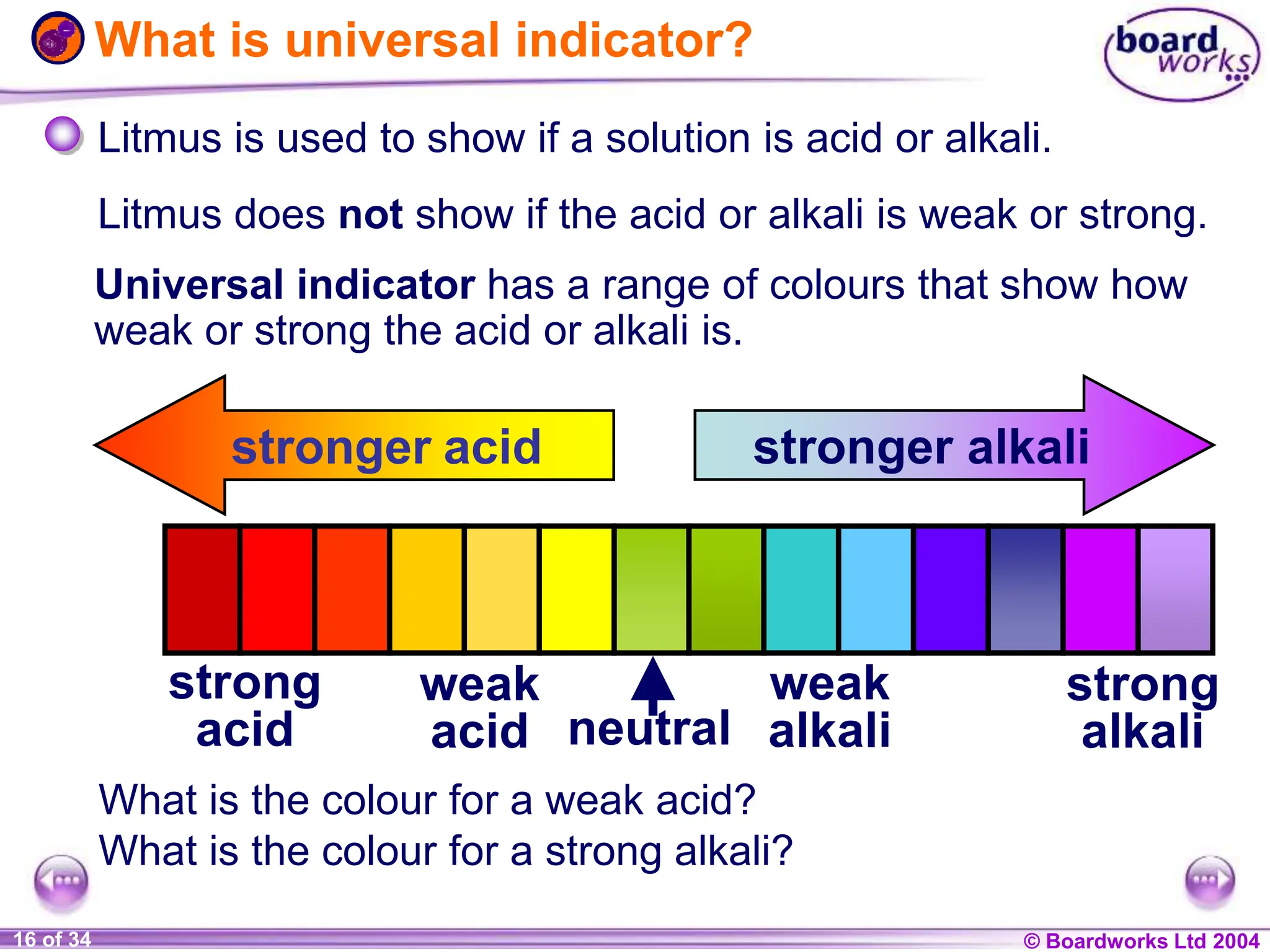
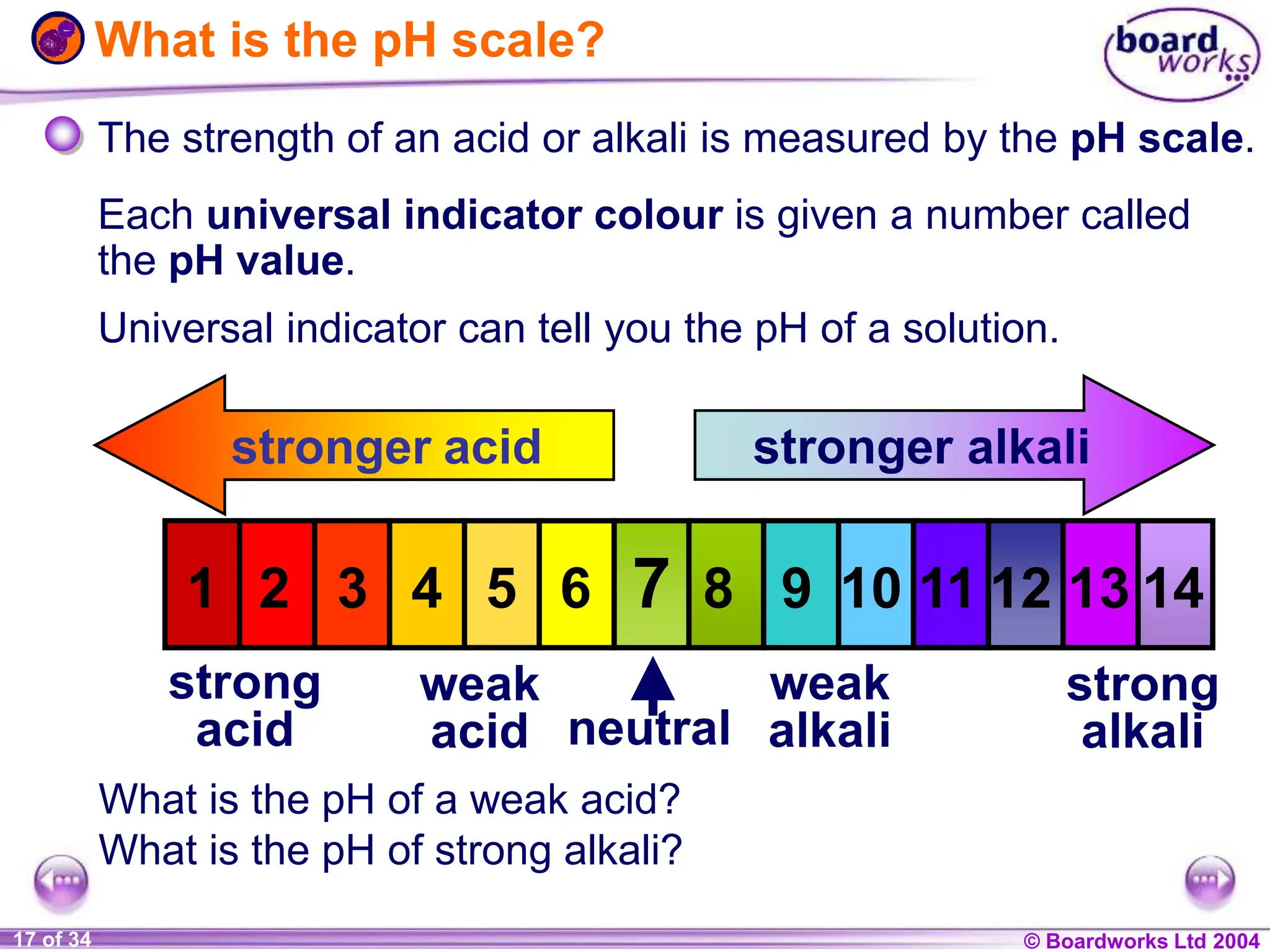
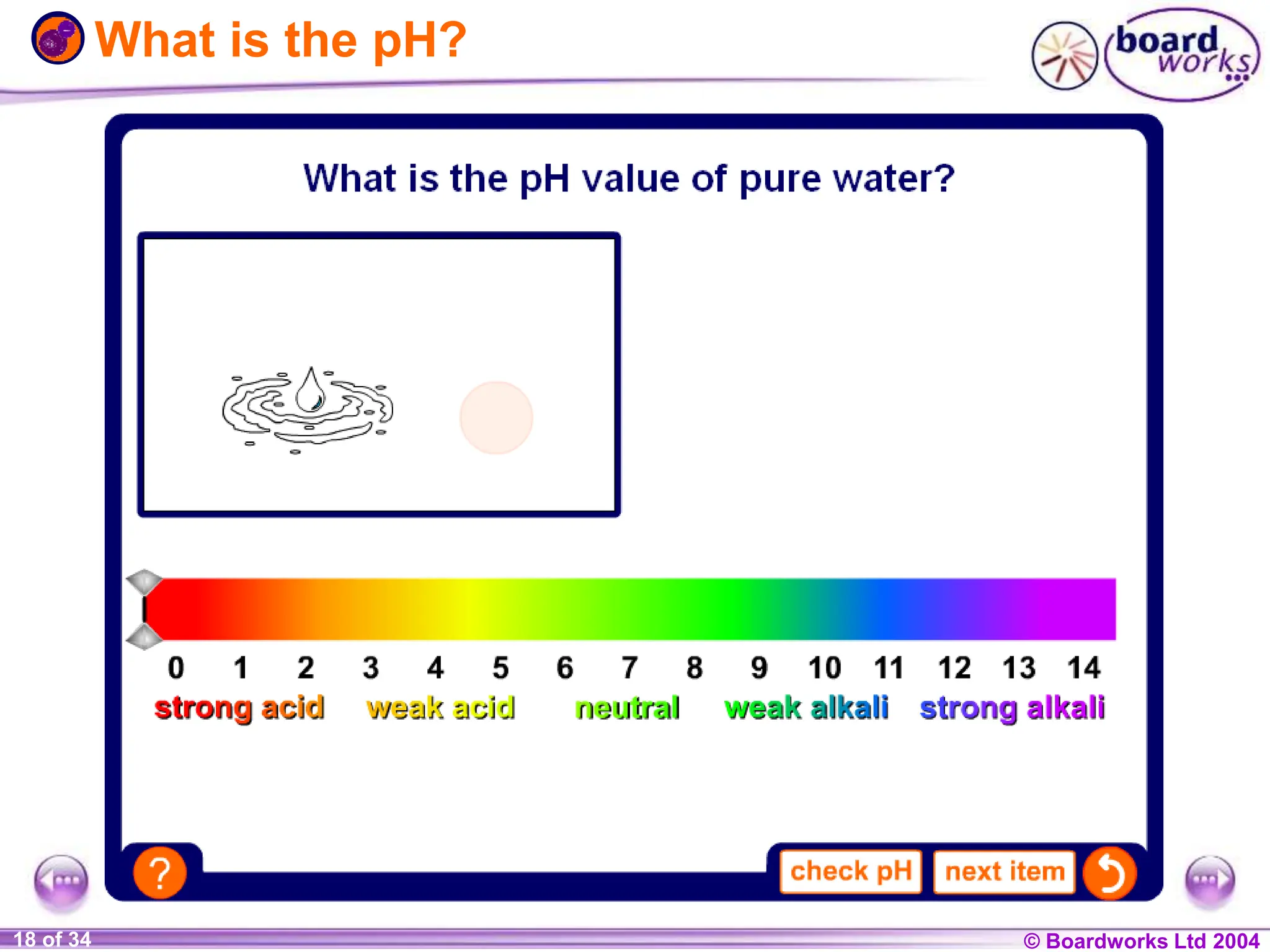
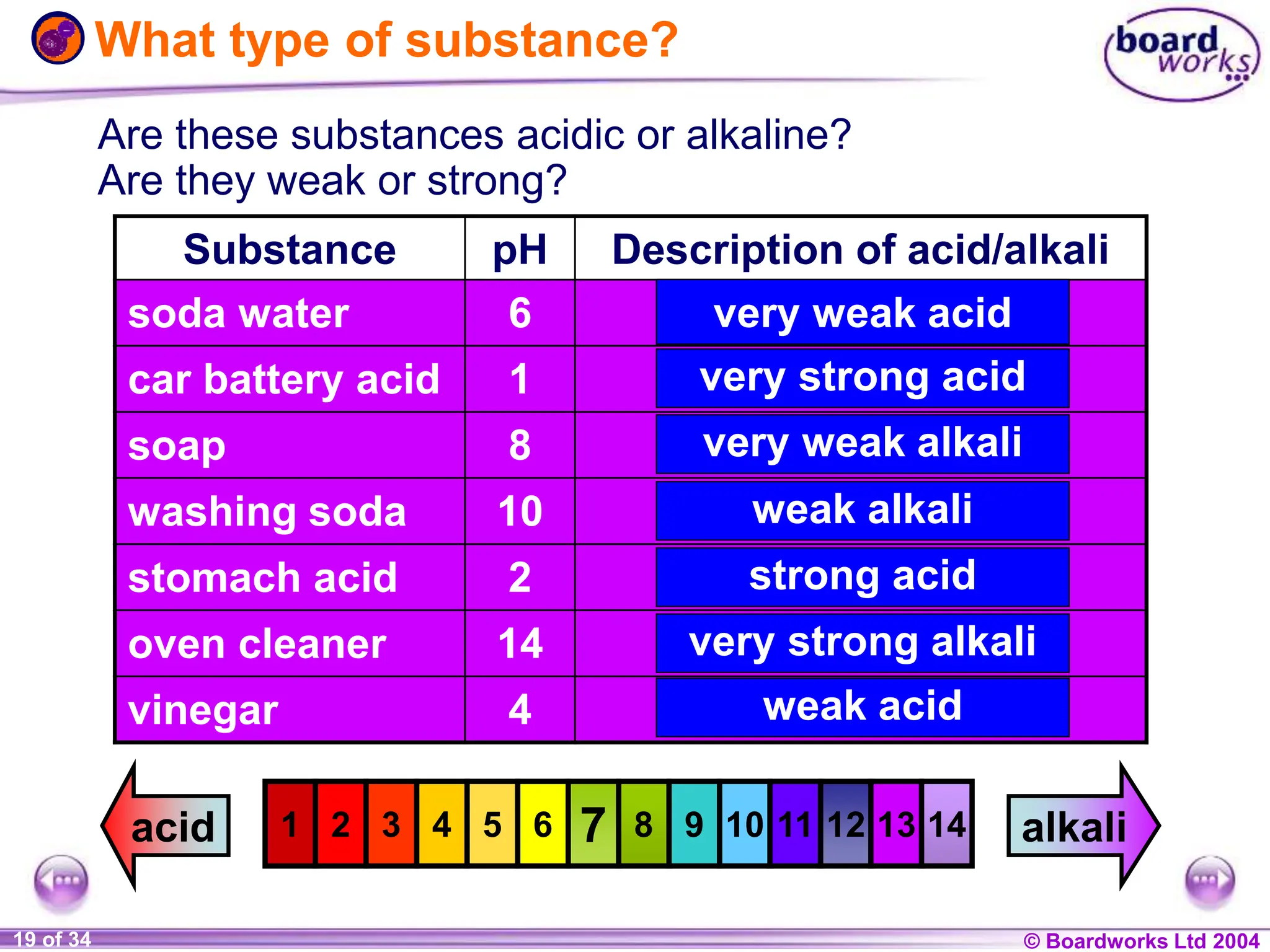
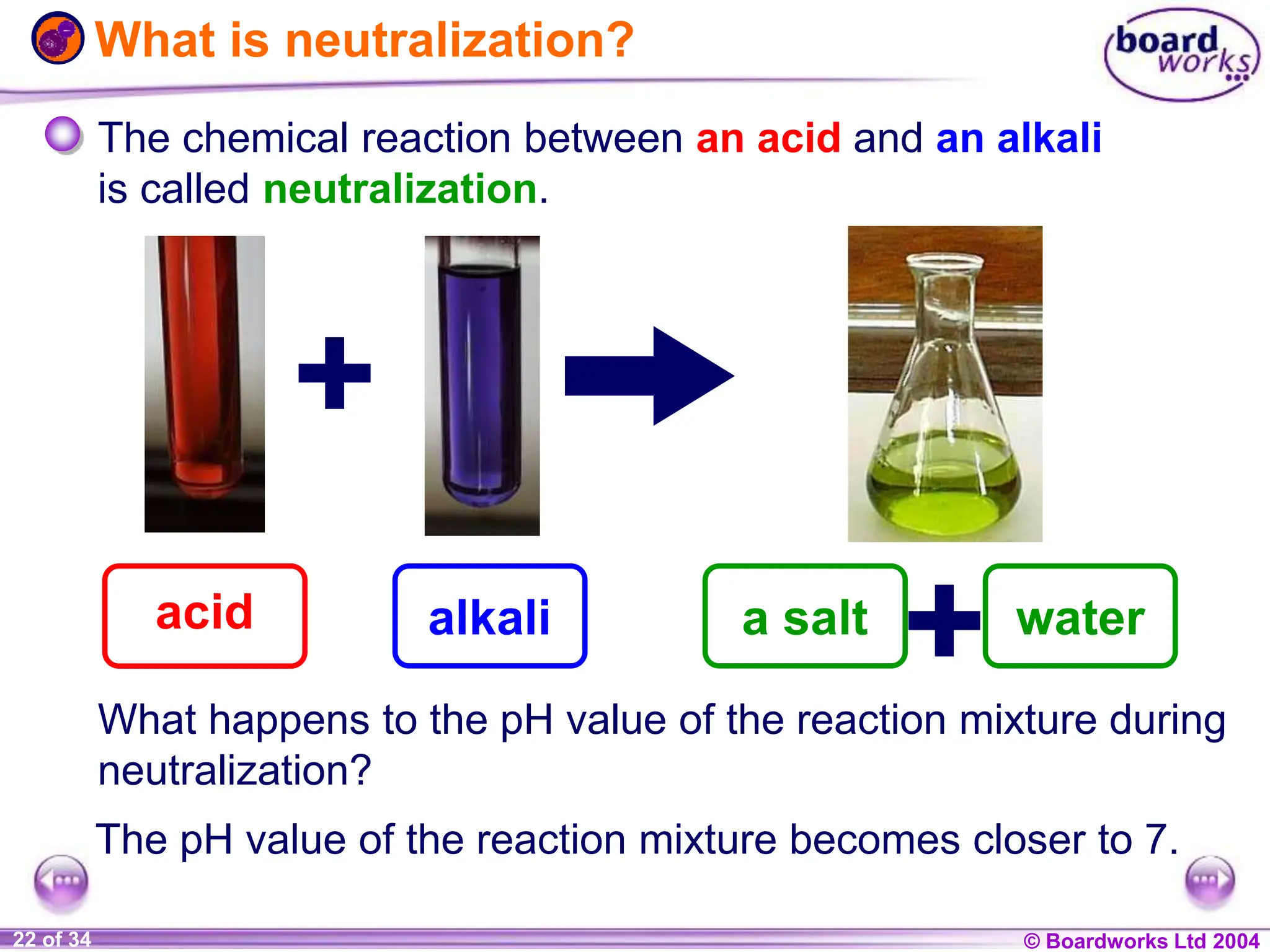
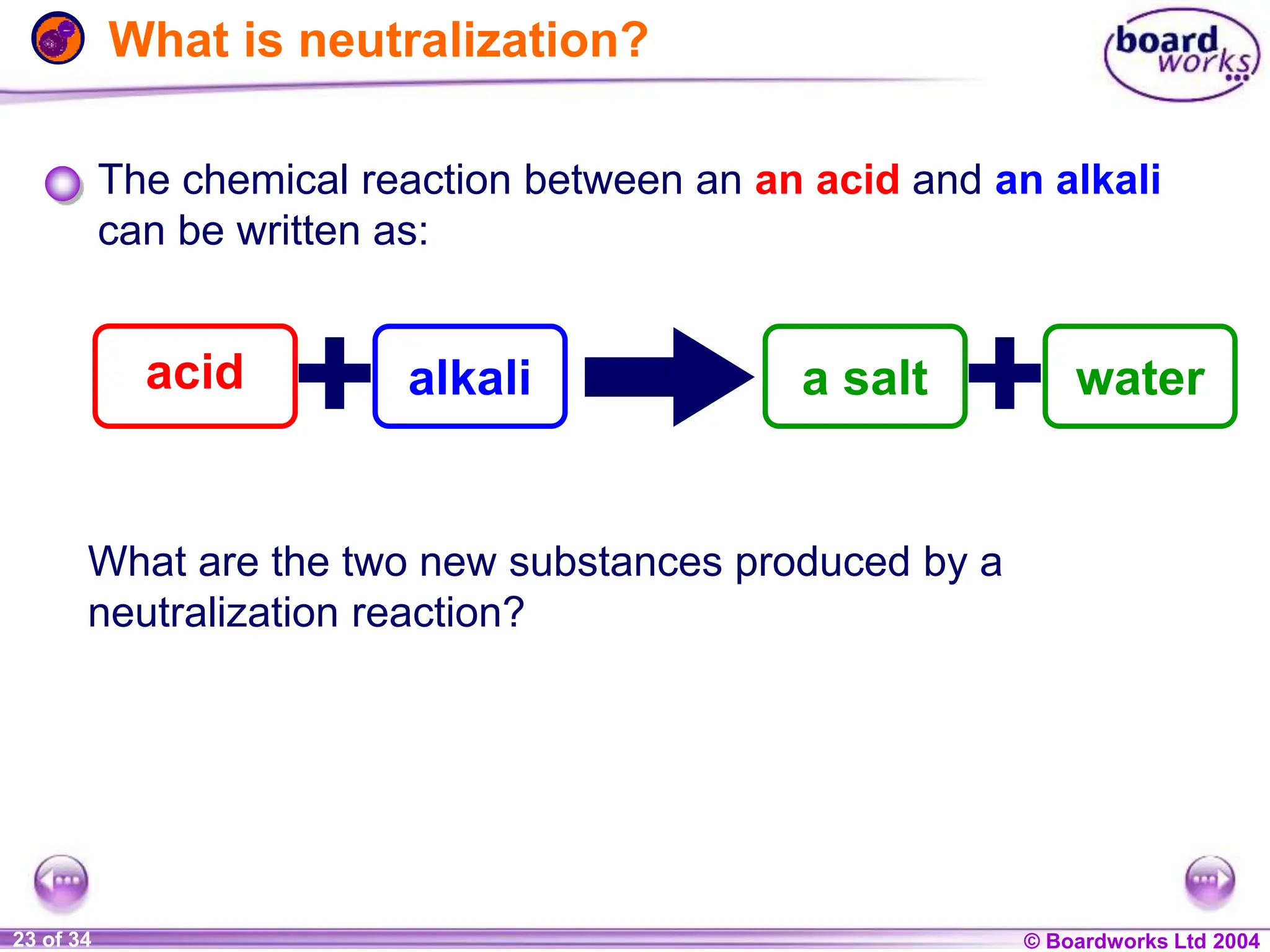
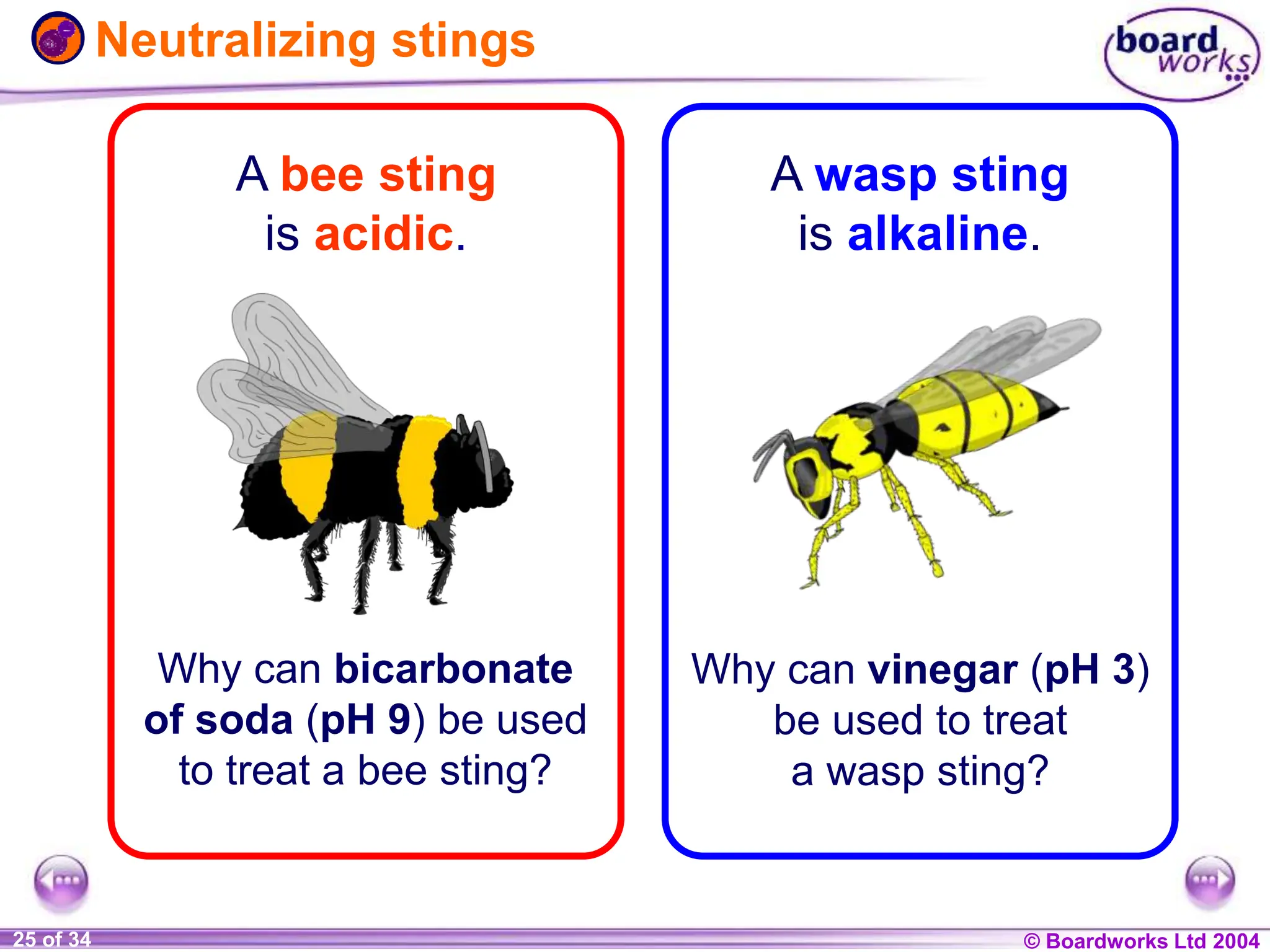
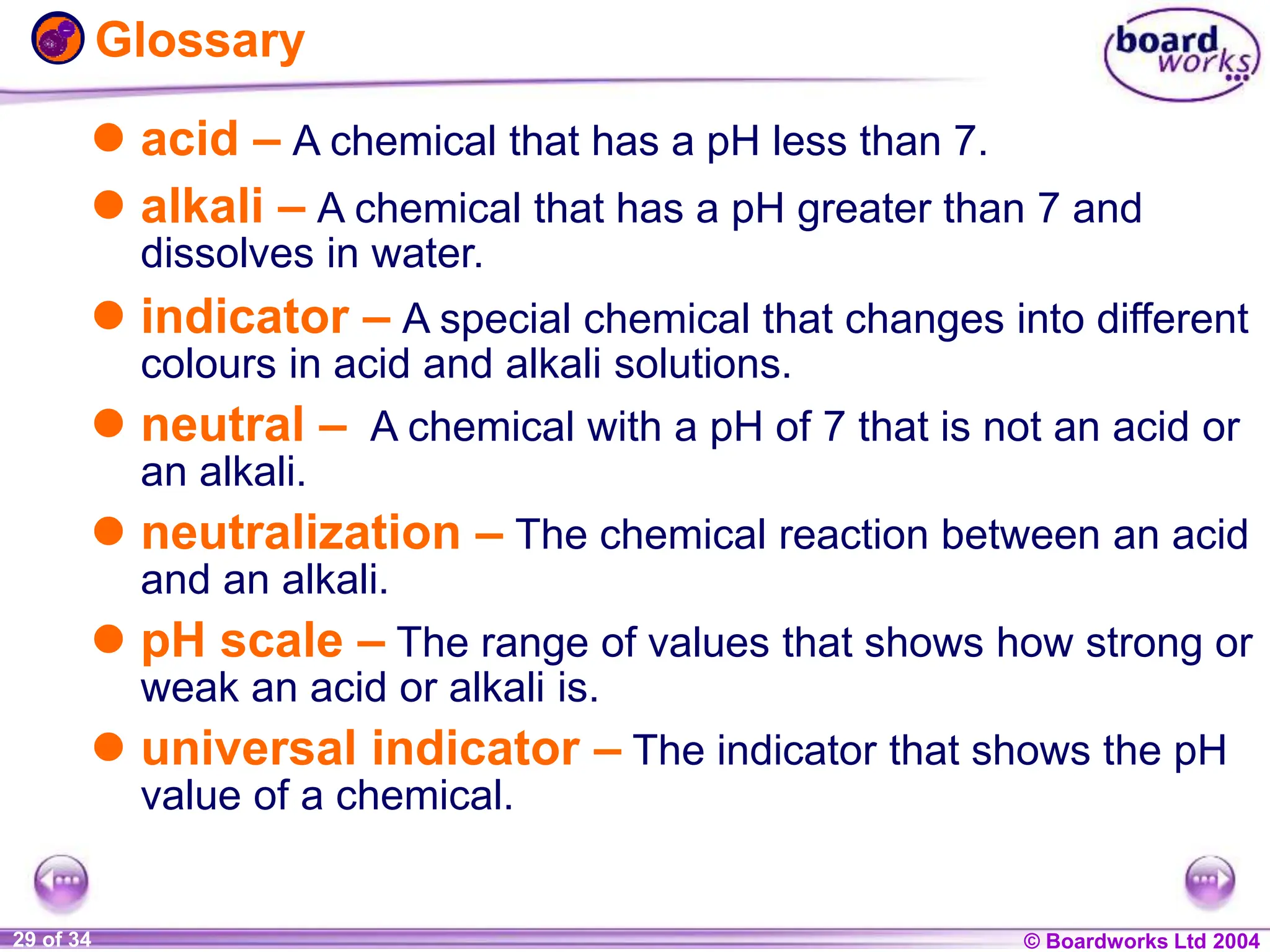
The document provides an overview of acids and alkalis, defining them as groups of chemicals with distinct properties, including pH levels and safety considerations. It discusses weak and strong variants, indicators used for testing, and the concept of neutralization, which describes the chemical reaction between acids and alkalis. Additionally, it highlights real-world applications and implications, such as the effects of acid rain and soil neutrality.