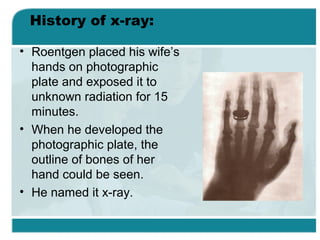
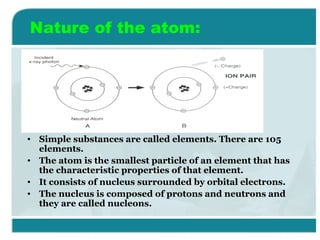
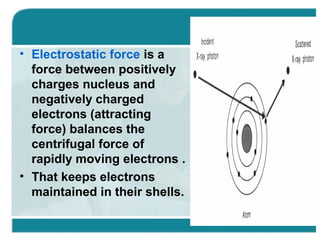
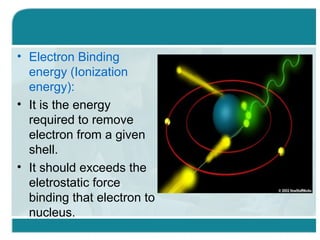
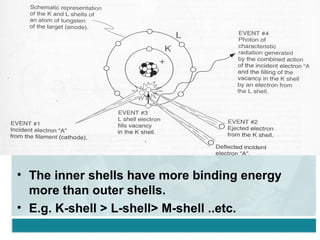
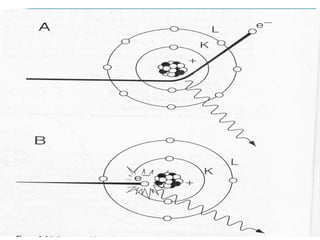
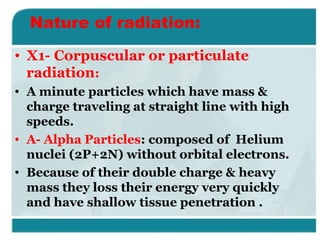
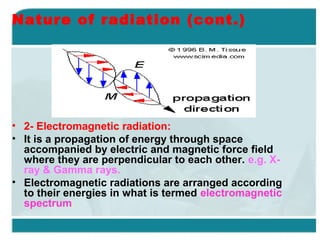
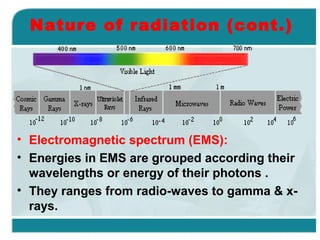
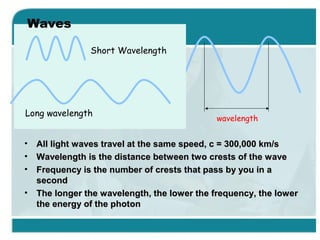
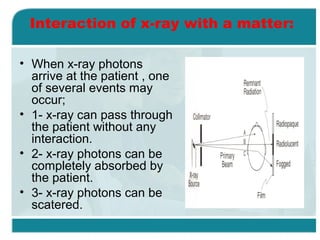
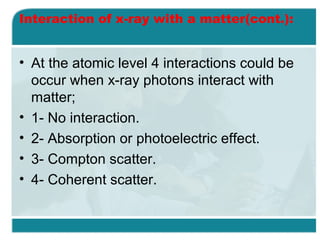
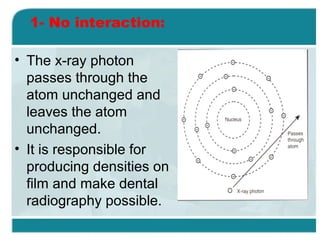
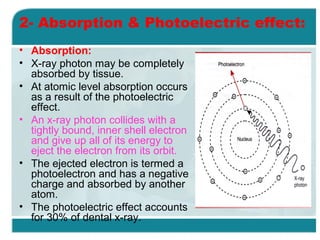
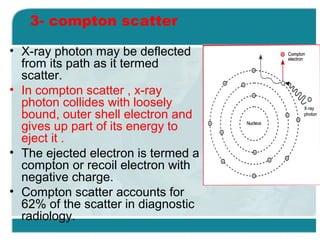
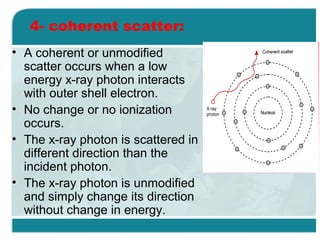
Wilhelm Conrad Röentgen was a German physicist who discovered x-rays in 1895. He was awarded the first Nobel Prize in Physics in 1901 for his discovery. Röentgen discovered x-rays accidentally while experimenting with cathode ray tubes. He noticed a fluorescent glow coming from a nearby screen and realized some new type of radiation was causing this. When he placed his wife's hand on photographic plates and exposed them to this radiation, the outline of bones in her hand appeared on the developed plate. This led him to name this new type of radiation "x-rays".