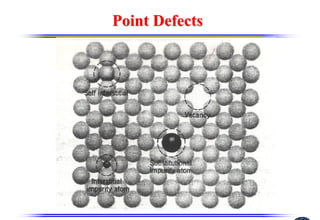
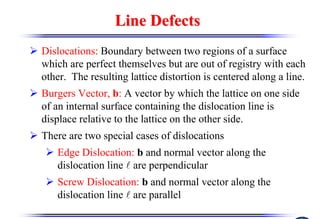
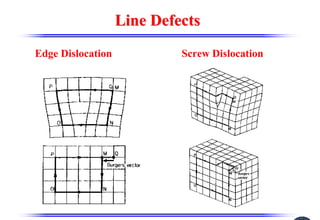
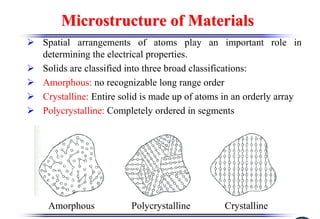
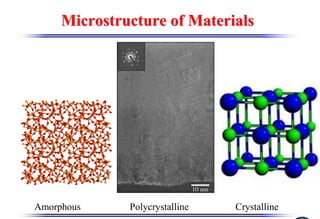
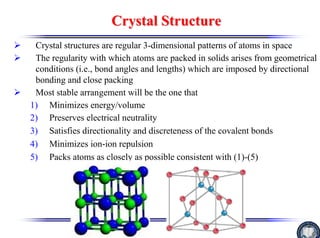
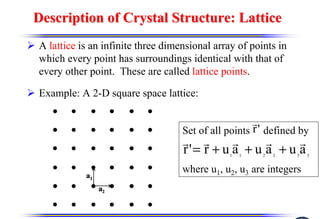
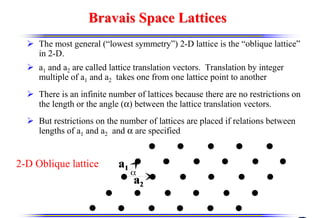
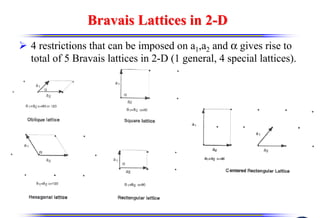
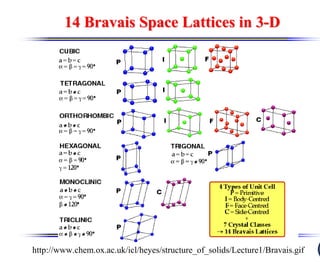
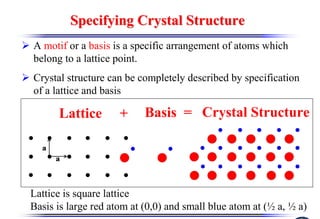
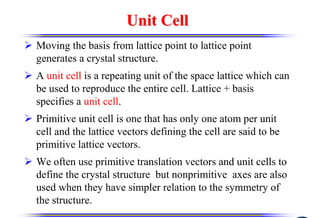
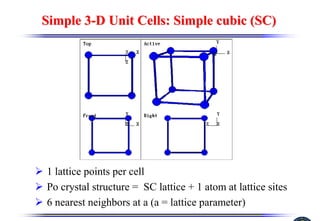
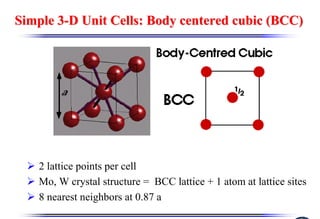
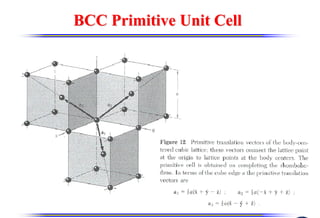
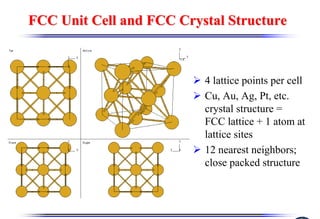
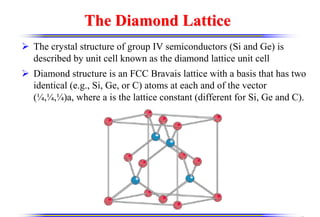
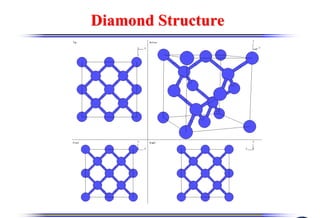
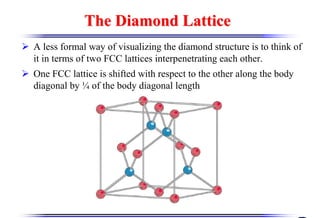
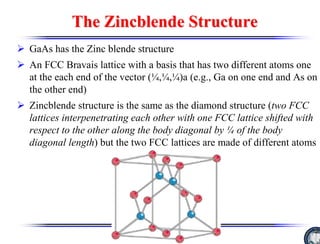
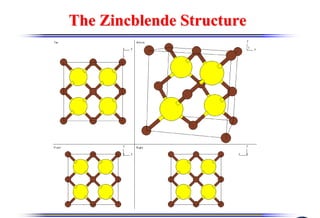
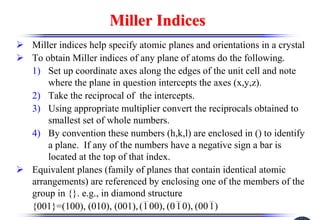
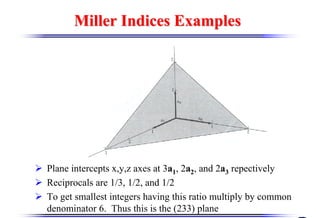
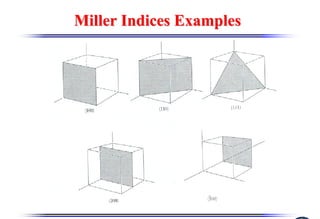
The document discusses crystal structure and defects. It begins by classifying materials as amorphous, polycrystalline, or crystalline based on their atomic structure. Crystalline materials have an orderly array of atoms described by a lattice and basis. Common crystal structures include simple cubic, body centered cubic, and face centered cubic. Defects in the crystal structure are also discussed, including point defects like vacancies and interstitials, and line defects like dislocations. Miller indices are used to describe planes and directions in crystal structures.

![Crystallographic Directions
Crystallographic Directions
¾ Miller indices enclosed within [ ] are used to designate
directions within a crystal
¾ Note also that the Miller indices also give the direction of
the normal vector of the plane they describe
¾ Miller indices enclosed within < > are used to designate
equivalent (family of) directions.
e.g., in simple cubic lattice [100] is the x-axis direction,
<100> describes all directions parallel to the x, y and z
axis.](https://image.slidesharecdn.com/crystalstructure-221212141046-c1152c8e/85/Crystal-structure-pdf-24-320.jpg)