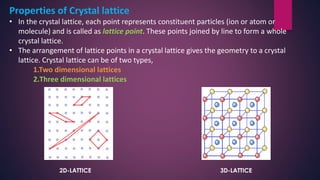
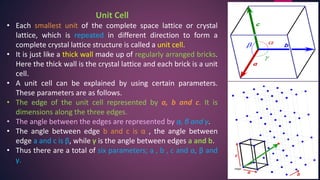
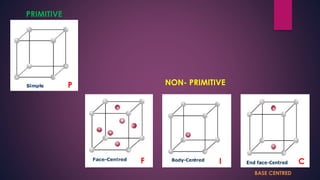
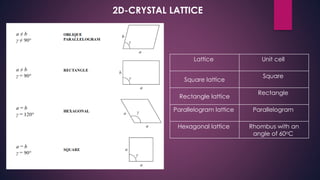
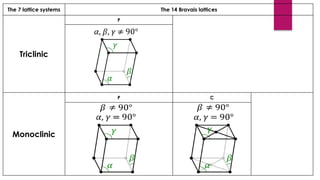
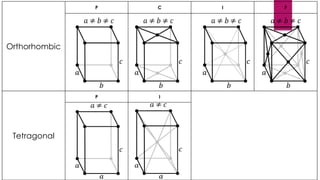
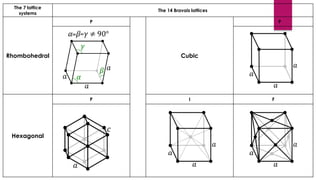
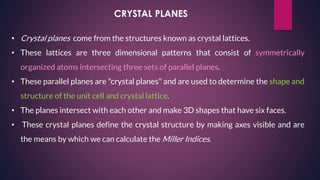
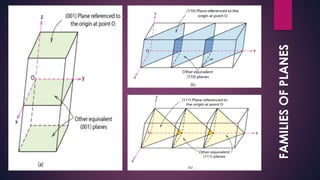
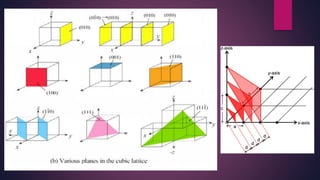
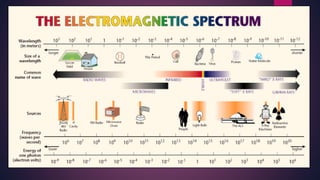
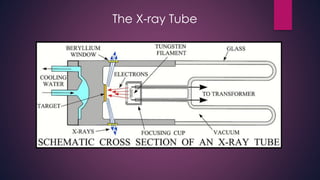
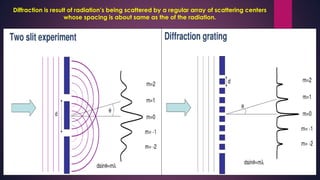
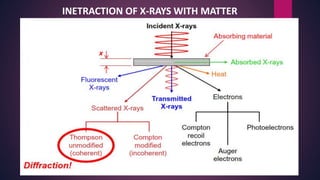
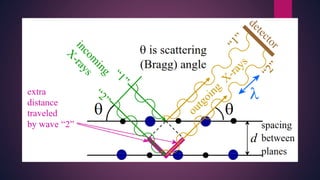
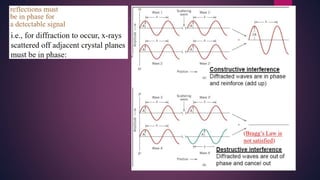
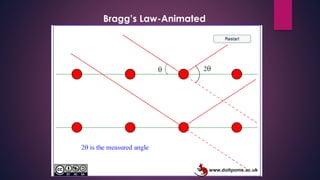
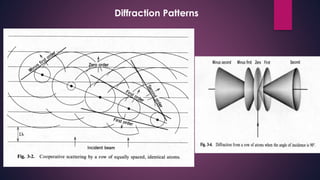
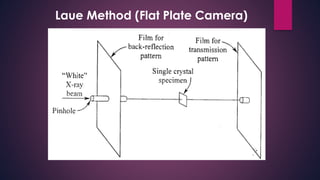
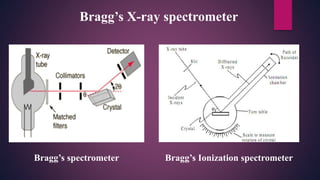
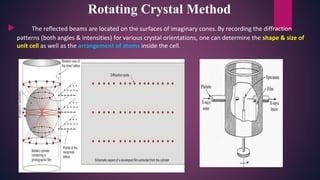
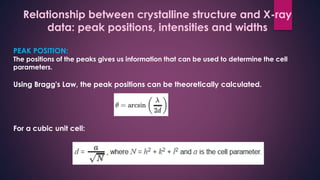
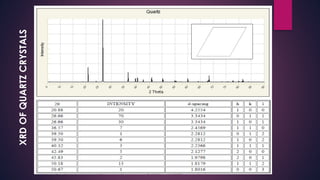
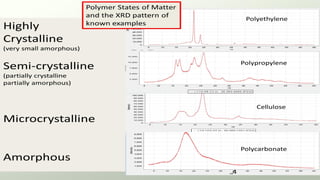
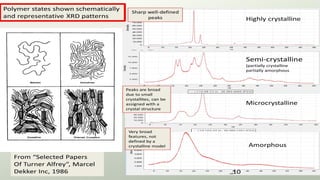
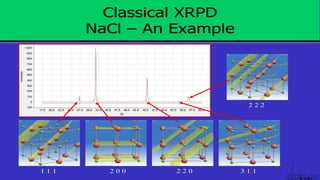
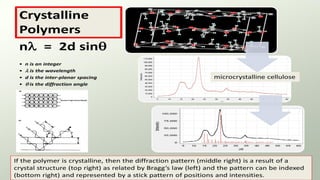
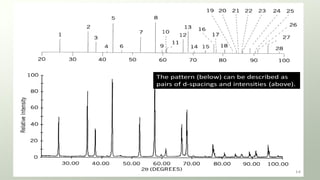
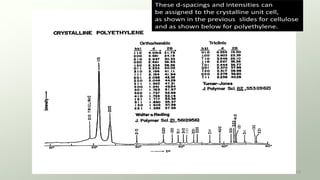
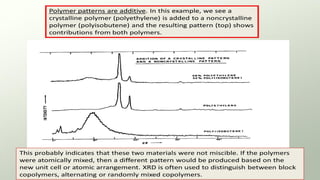
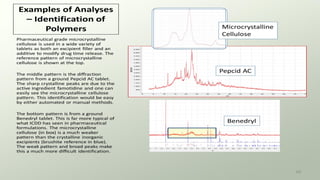
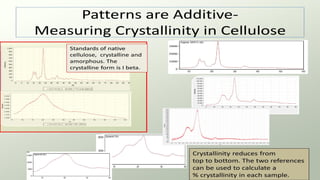
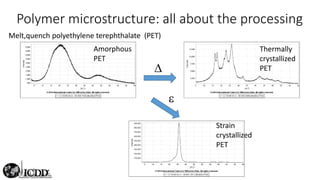
The document provides an overview of elementary crystallography and X-ray diffraction (XRD), detailing the classification of solids into crystals and amorphous materials based on their structural organization. It discusses crystal symmetries, lattice structures, and the use of Miller indices for expressing crystal orientations, as well as the historical development and application of X-ray diffraction techniques for analyzing crystal structures. XRD is highlighted as a non-destructive method for determining various properties of crystalline materials, including their structure, orientation, and crystallinity.