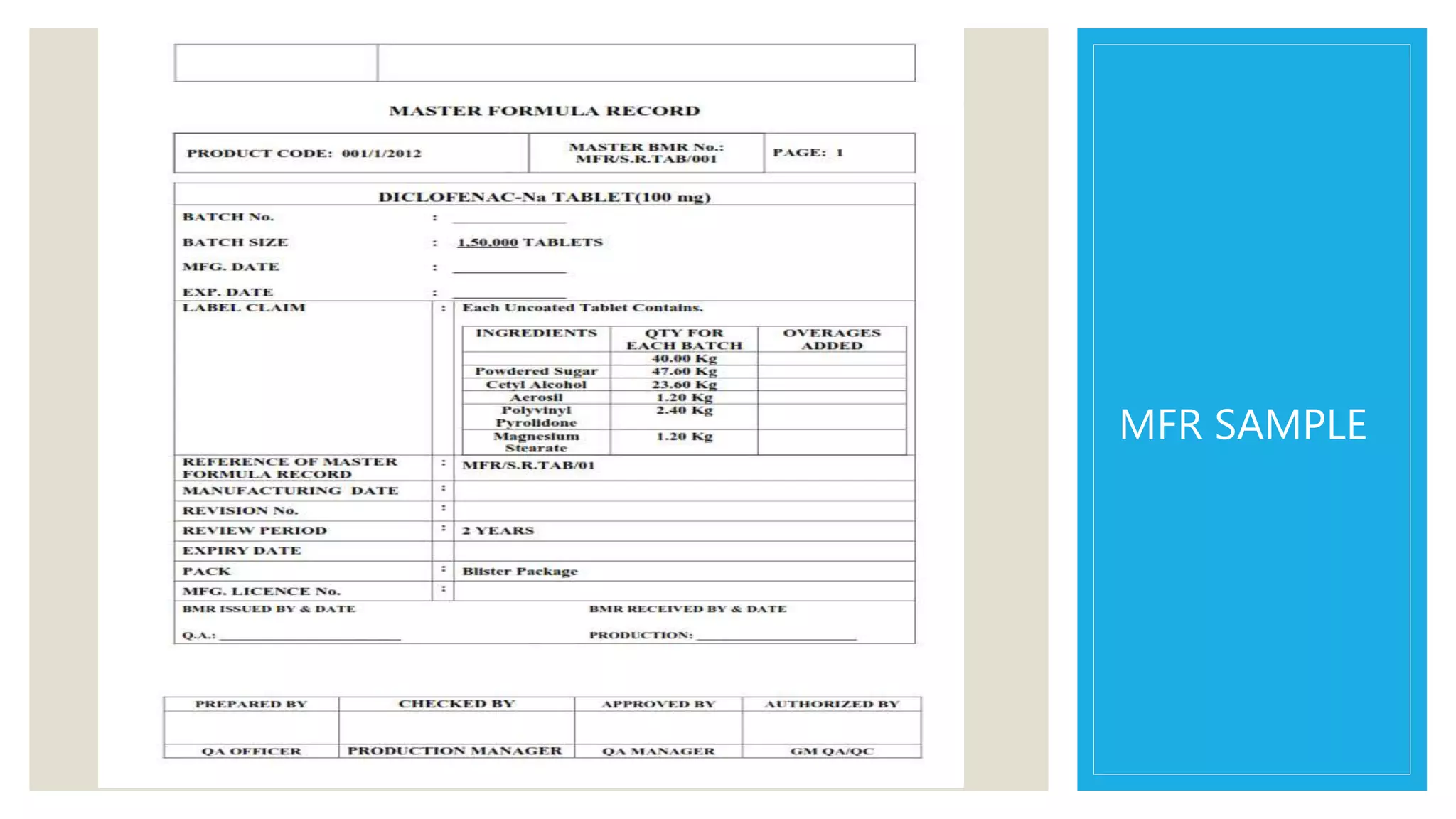
The document outlines the role and functions of regulatory affairs in the pharmaceutical industry, focusing on the interface with drug regulatory authorities, such as the FDA and CDSCO, to ensure the safety, efficacy, and quality of drugs. It discusses essential components like the Master Formula Record and Drug Master File, as well as the processes for New Drug Applications (NDA) and Abbreviated New Drug Applications (ANDA). Additionally, it highlights the significance of bioequivalence studies, the Hatch-Waxman Act, and post-marketing surveillance in drug development and approval.