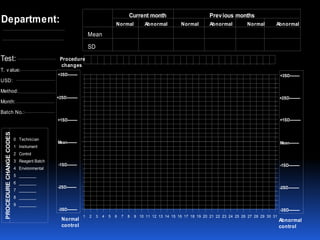
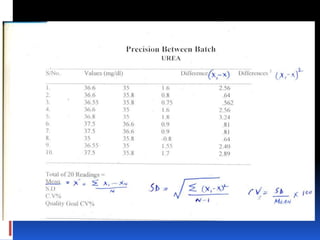
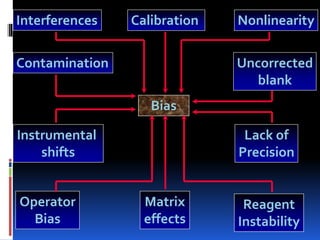
This document discusses general principles of control charts used to monitor analytical testing methods. It provides information on how control charts are constructed using mean and standard deviation values from repeated measurements on known specimens. Control limits are calculated and plotted, with points falling within the limits indicating proper method performance. The document also describes procedures for identifying random and systematic errors using rules for interpreting control chart results.