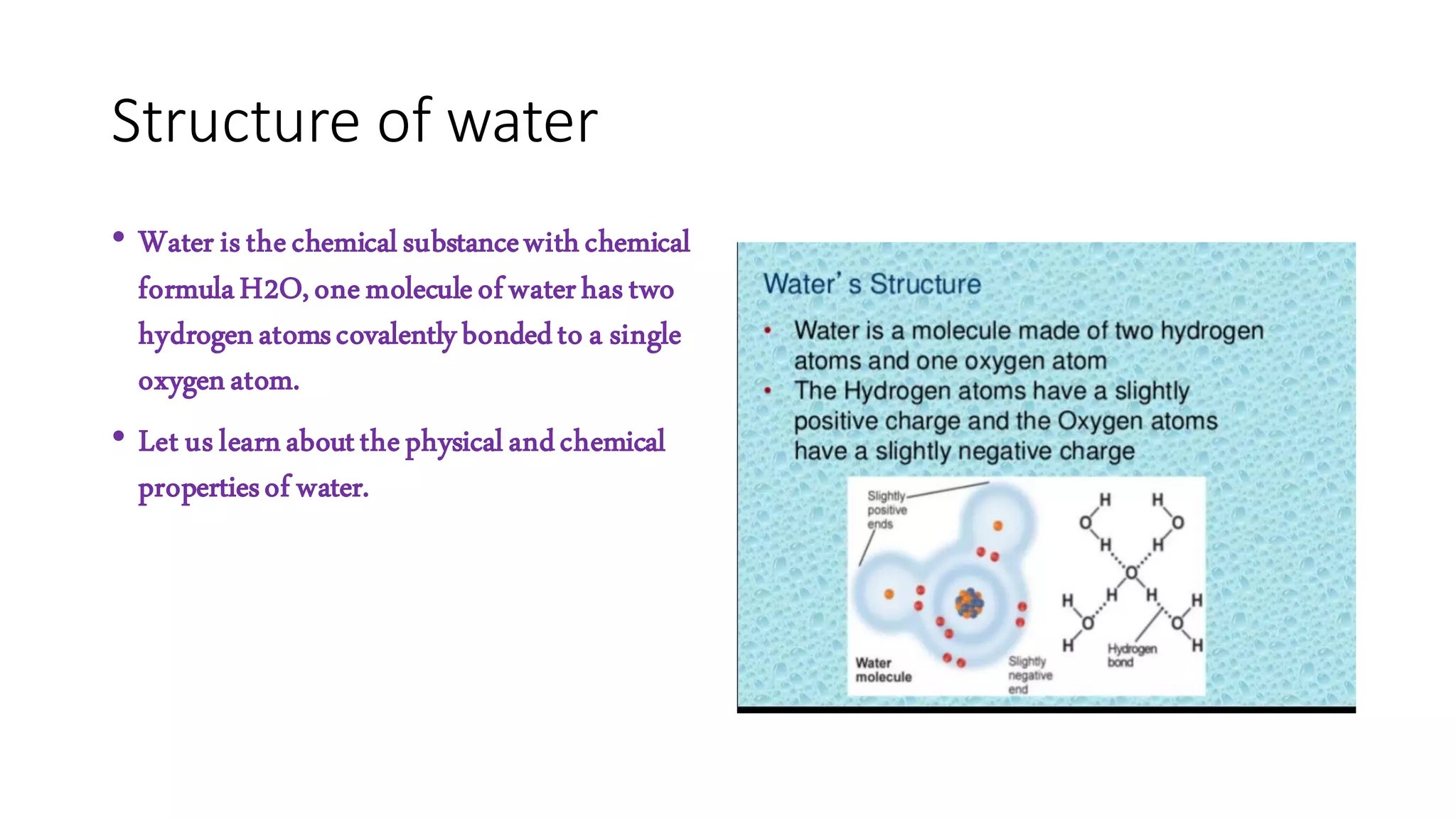
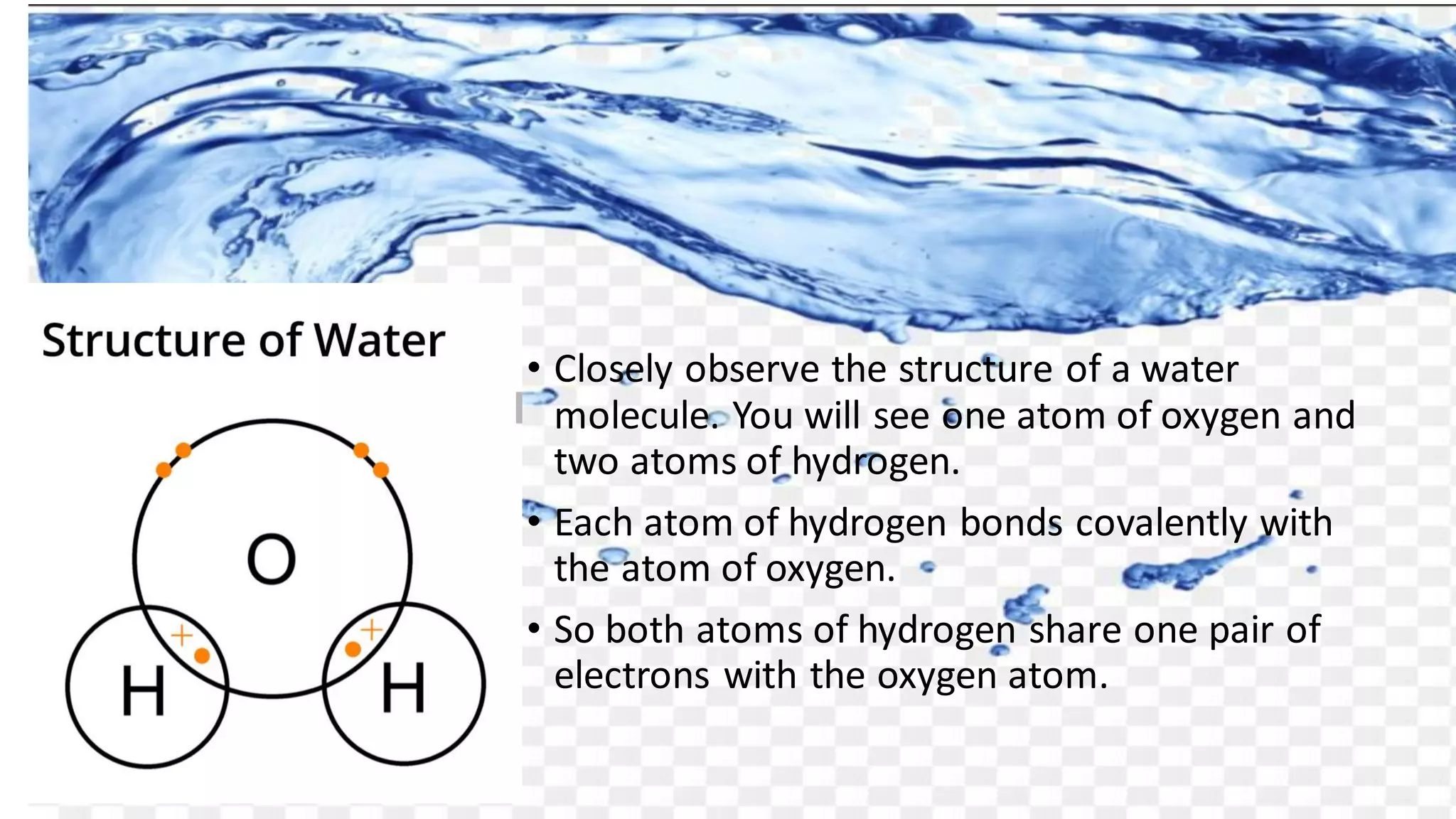
Water has a chemical formula of H2O, with two hydrogen atoms covalently bonded to a single oxygen atom. This gives water an angular, bent structure that is polar. Water's polarity allows it to form hydrogen bonds between molecules. These hydrogen bonds give water unusual properties like high melting and boiling points. Water is also an excellent solvent due to its polarity. These unique properties make water essential for life on Earth and allow it to perform important functions in biological systems like transport and metabolic reactions.