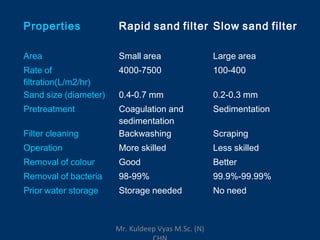
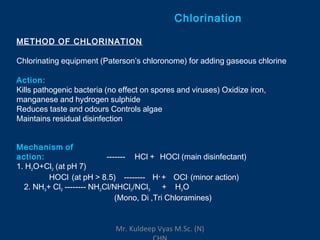
Three sentences:
The document discusses various methods for water purification at large and small scales, including storage, filtration using slow sand filters and rapid sand filters, and disinfection using chlorination, sunlight, and other chemical disinfectants. Large scale purification involves storage, filtration, and disinfection while small scale domestic purification focuses on boiling, chemical disinfection, and household filtration using ceramic or other filters. Proper purification removes pathogens and improves water quality for drinking.