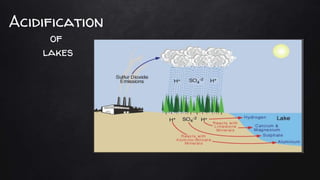
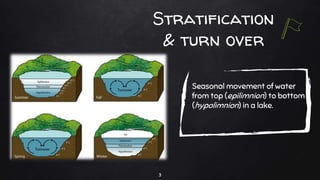
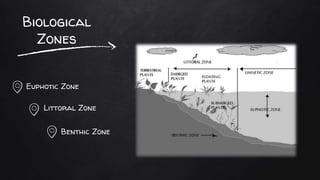
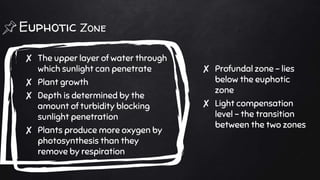
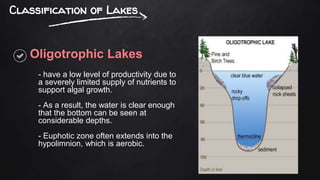
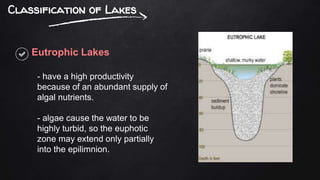
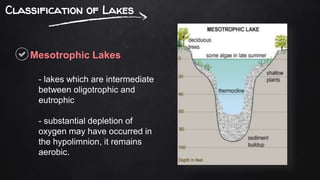
The document discusses water pollution in lakes, focusing on limnology, lake stratification, and productivity as well as the classification of lakes into oligotrophic, mesotrophic, eutrophic, and senescent. It explains the processes of natural and cultural eutrophication, the nutrient requirements for algal growth, and the impact of acidification on lake ecosystems. Solutions to mitigate acidification include liming and reducing air pollution to prevent acid rain.















![N itrogen
✘ Usually in the form of nitrates [NO3
−
]
✘ Converting nitrogen gas to organic
nitrogen though nitrogen-fixing bacteria
such as cyanobacteria
✘ Comes from external sources by way of
inflowing streams or groundwater
✘ Aerobic
○ When taken for algal growth, forms
amino-nitrogen NO2
−
○ When algae dies, forms ammonia
[NH3 ]
✘ Anaerobic
○ Nitrate is reduced to [N2] through
denitrification](https://image.slidesharecdn.com/water-pollution-in-lakes-190109163416/85/Water-Pollution-in-Lakes-23-320.jpg)
![P hosphorus
✘ Originates from external
sources
✘ Taken up by algae in the
inorganic form [PO4
3−
] and
incorporated into organic
compounds
✘ When algae dies, returns to
its inorganic form](https://image.slidesharecdn.com/water-pollution-in-lakes-190109163416/85/Water-Pollution-in-Lakes-24-320.jpg)