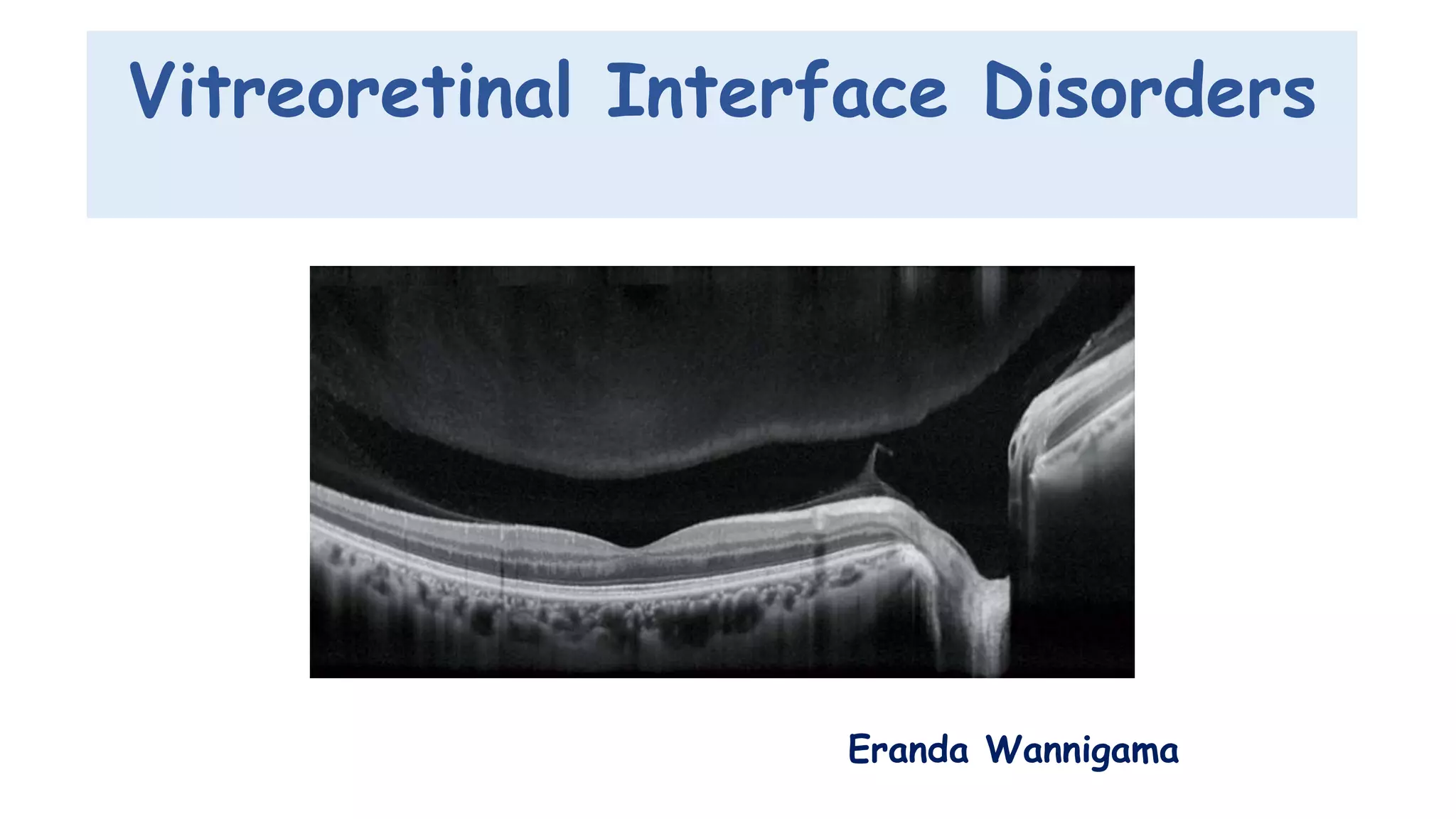
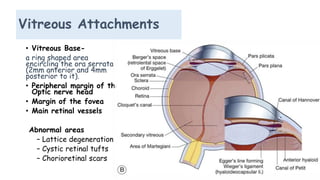
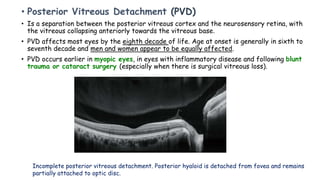
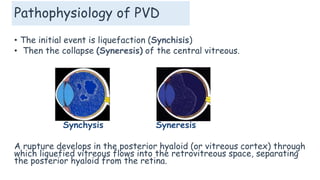
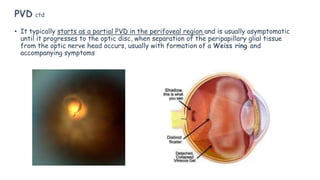
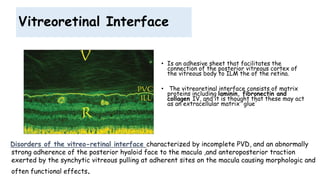
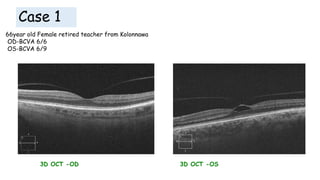
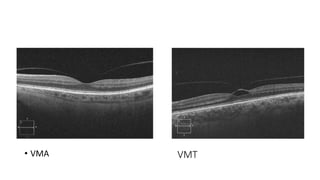
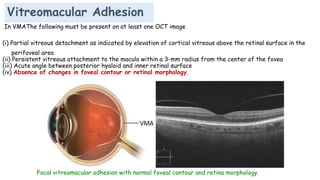
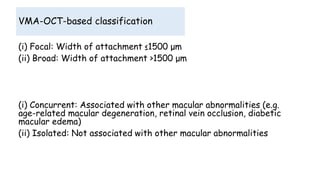
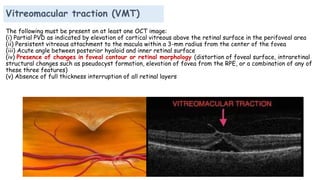
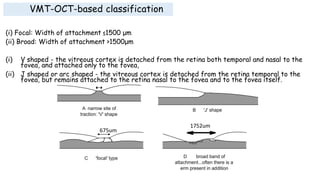
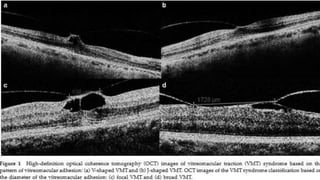
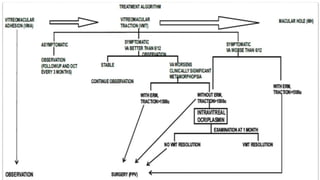
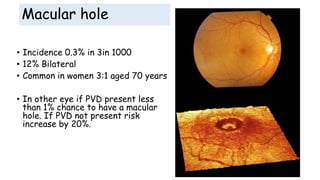
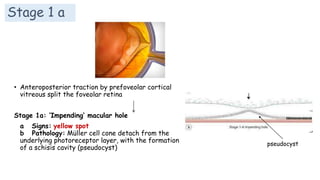
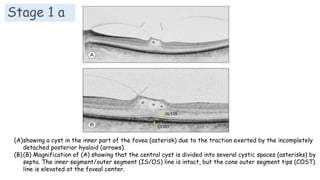
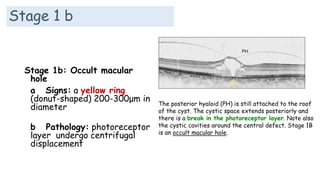
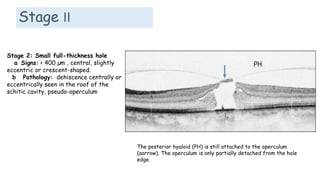
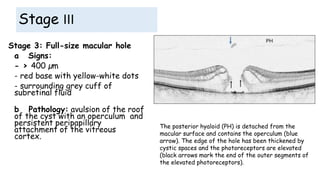
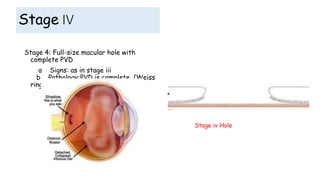
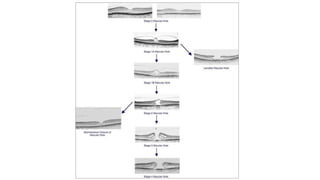
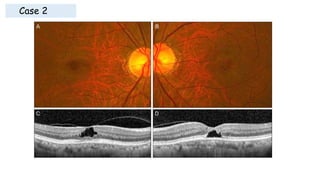
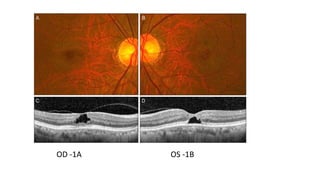
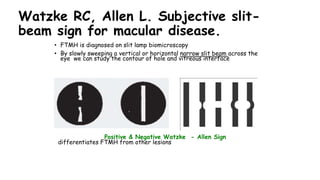
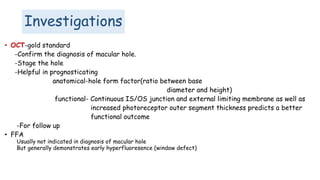
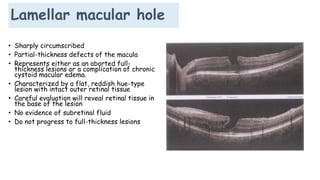
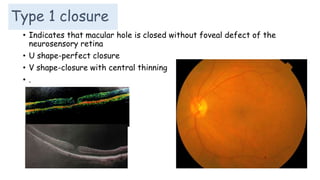
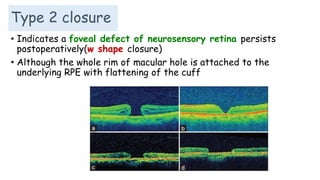
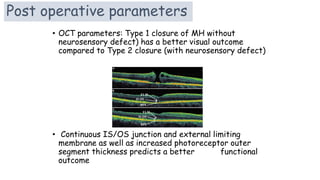
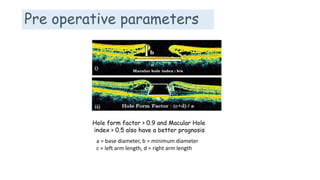
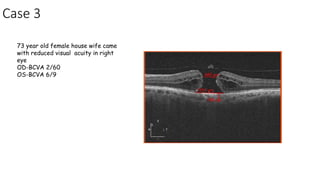
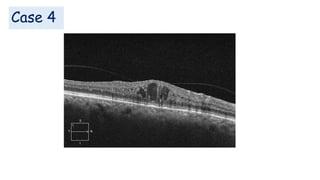
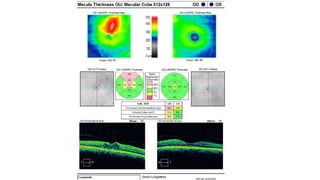
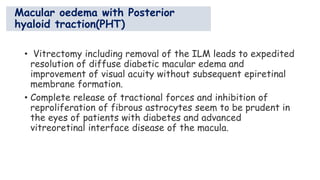
Vitreoretinal interface disorders involve abnormal adherence between the vitreous gel and the retina. Posterior vitreous detachment normally occurs in older individuals as the vitreous liquefies and separates from the retina. Vitreomacular adhesion and traction occur when the vitreous remains abnormally attached to the macula, which can cause macular edema or hole formation. Optical coherence tomography is useful for diagnosing and monitoring these disorders. Treatment may involve observation, pharmacologic vitreolysis to release attachments, or vitrectomy surgery to remove tractional forces on the macula.