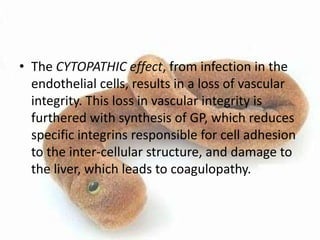
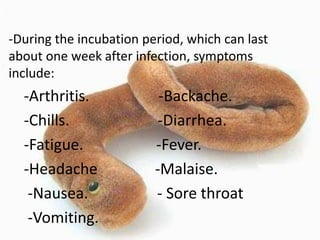
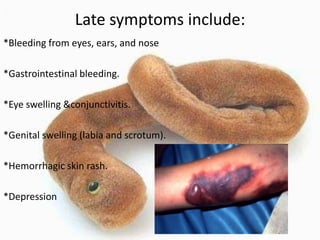
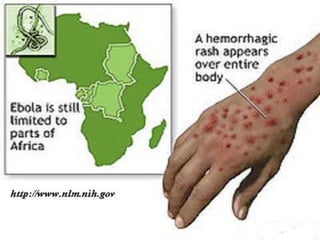
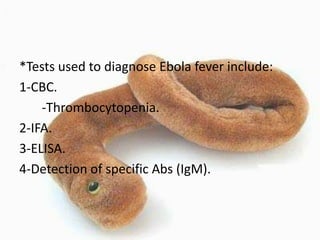
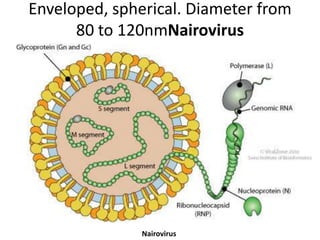
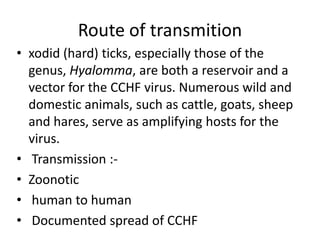
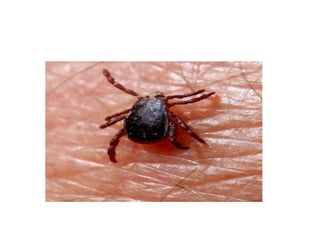
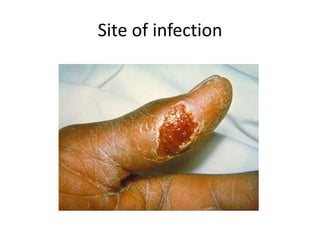
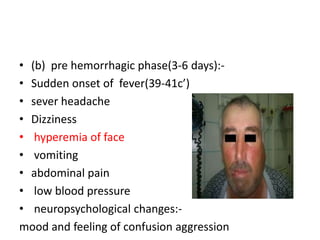
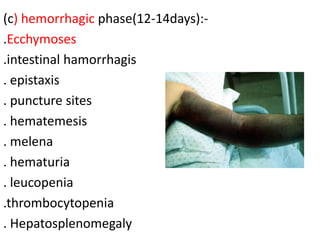
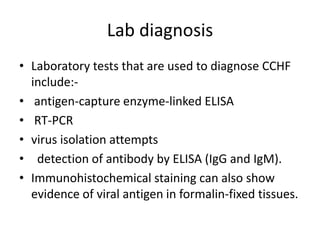
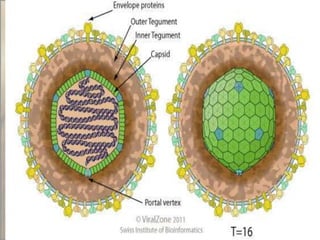
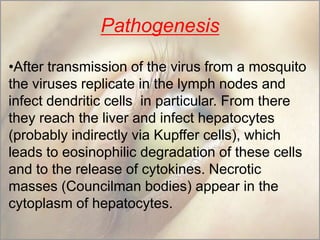
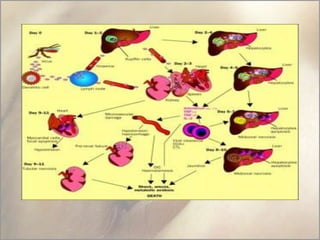
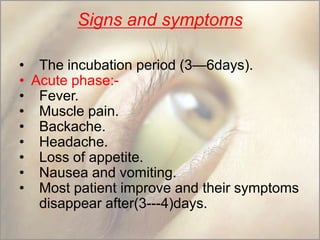
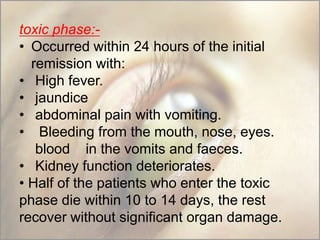
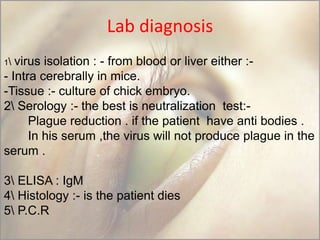
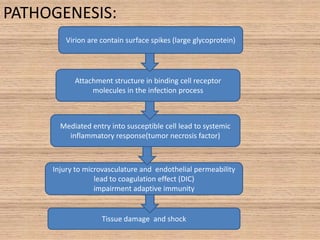
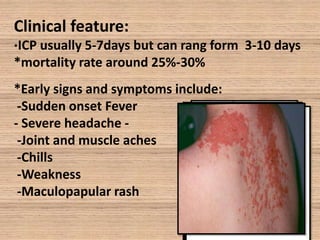
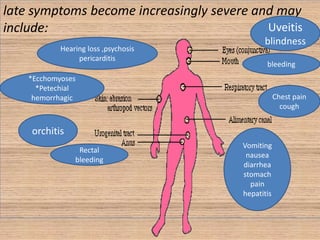
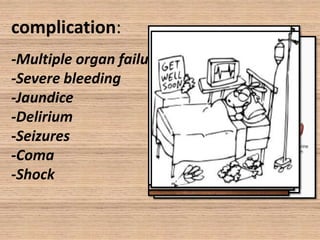
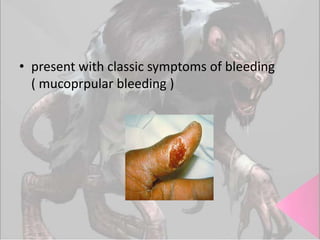
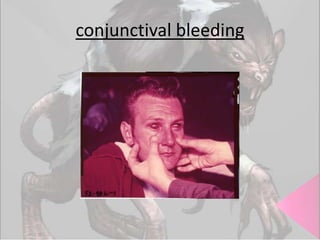
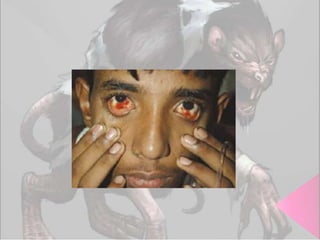
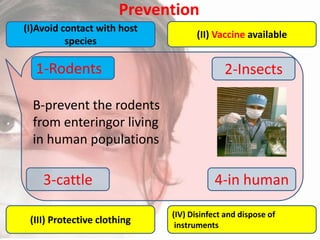
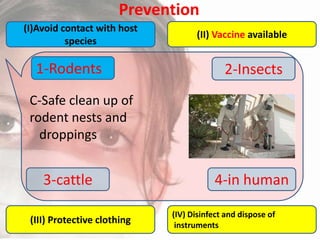
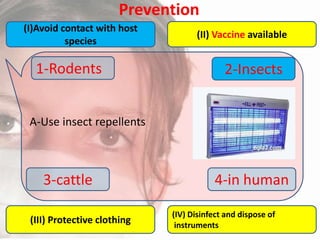
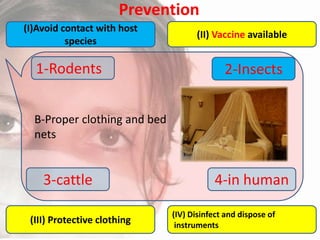
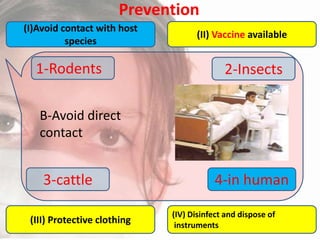
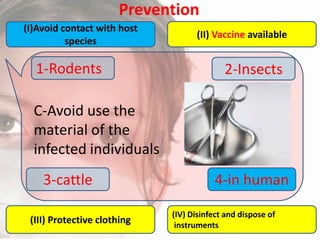
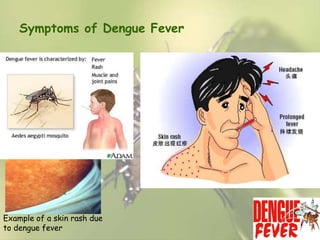
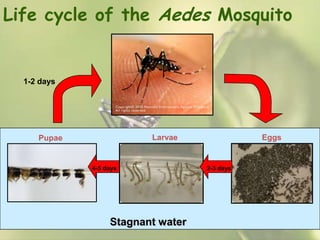
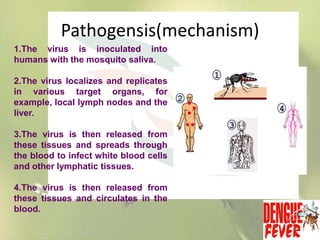
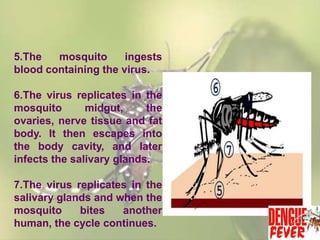
This document provides an overview of dengue fever. It begins with definitions of dengue fever and dengue virus. It describes the symptoms of dengue fever and characteristics of the Aedes mosquito, including its physical features, daytime biting habits, and egg laying in clean stagnant water. The life cycle of the Aedes mosquito is outlined, from egg to larva to pupa to adult. It explains how the Aedes mosquito transmits dengue virus between humans when taking blood meals. Pathogenesis and methods for laboratory diagnosis are briefly discussed. The document concludes with describing supportive treatments for dengue fever and emphasizing the importance of preventing Aedes mosquitoes from breeding to control the spread of the disease.













































































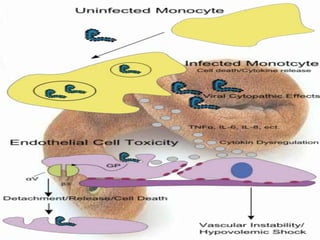
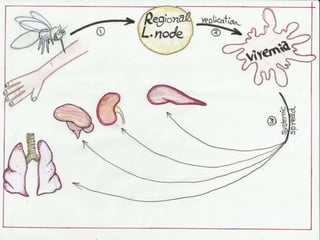
![• The sGP forms a dimetric complex which
interferes with the signaling of neutrophils, ,
which allows the virus to evade the immune
system by inhibiting early steps of neutrophil
activation. These white blood cells also serve as
carriers to transport the virus throughout the
entire body to places such as the lymph nodes,
liver, lungs, and spleen.] The presence of viral
particles and cell damage resulting from causes
the release of cytokines (specificallyTNF , IL 6, IL
8, etc.), which are the signaling molecules for
fever and inflammation.](https://image.slidesharecdn.com/hemorrhagicfevercommunti-140127121806-phpapp01/85/Hemorrhagic-fever-communti-108-320.jpg)