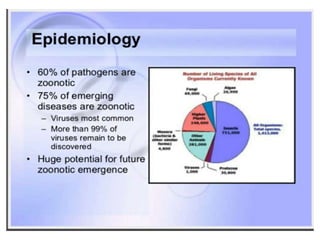
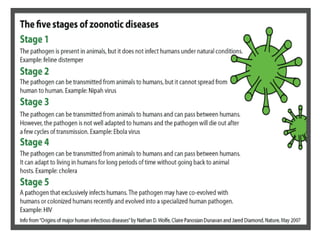
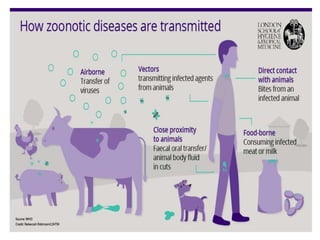
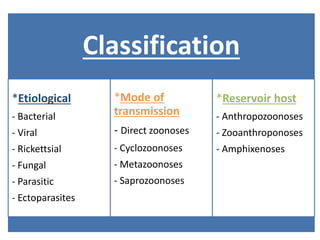
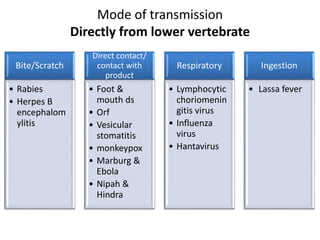
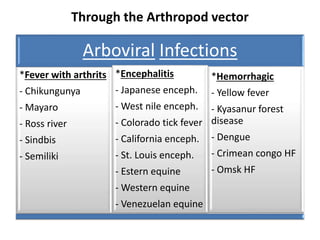
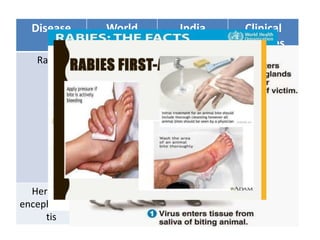
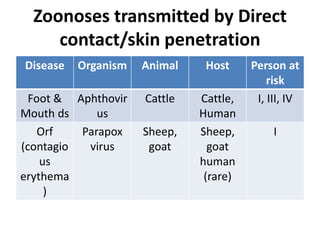
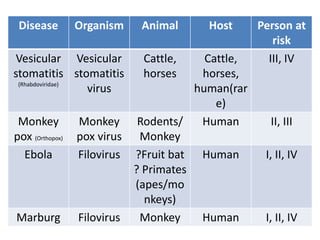
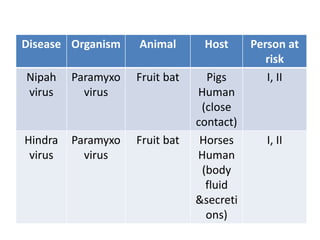
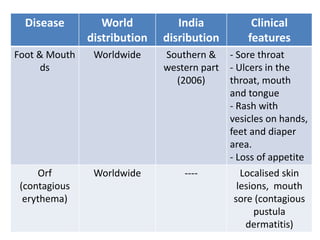
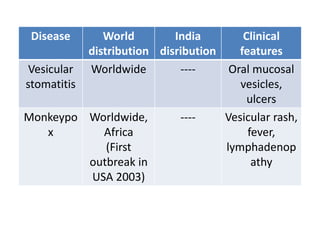
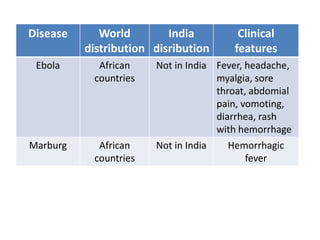
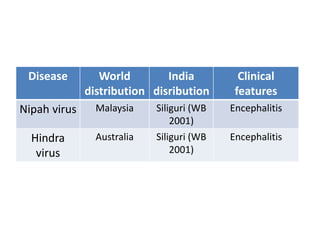
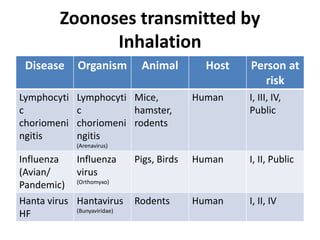
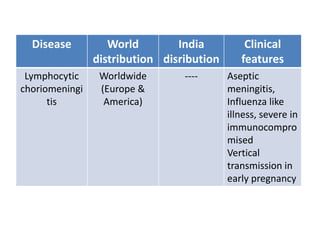
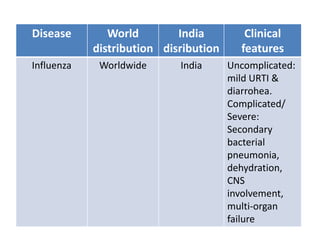
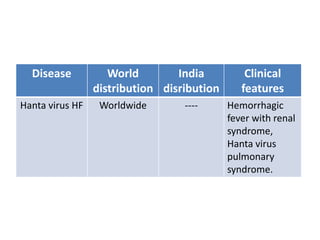
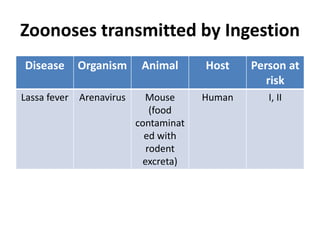
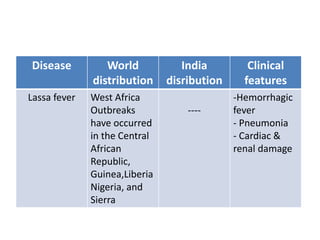
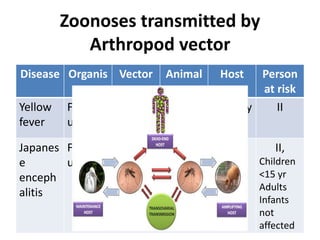
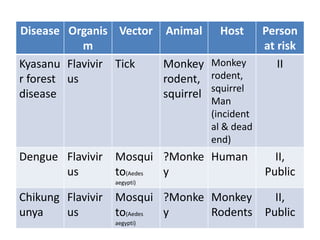
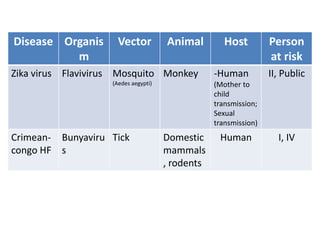
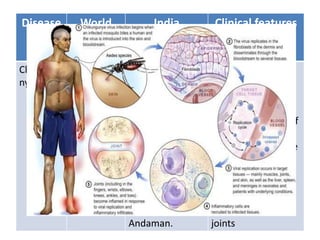
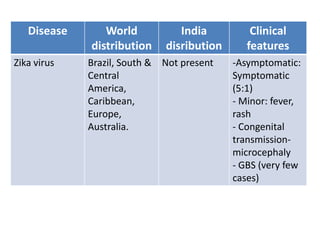
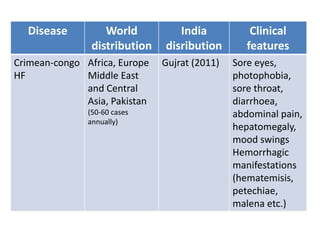
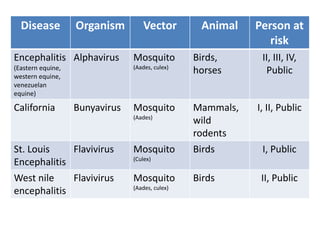
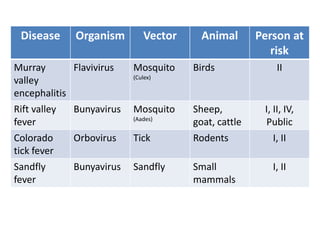
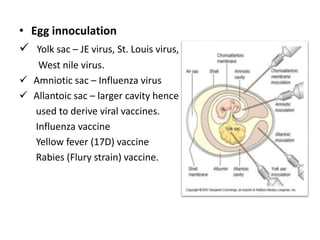
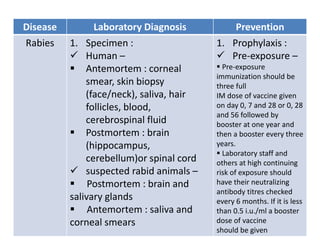
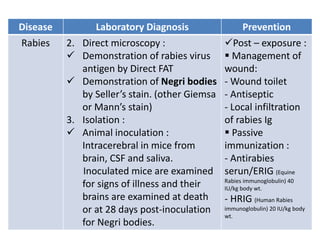
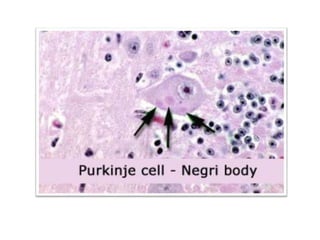
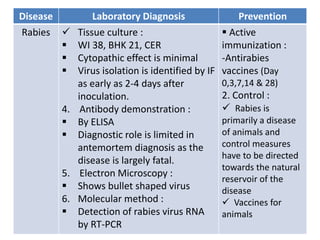
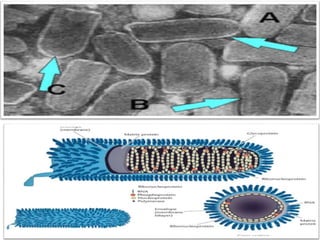
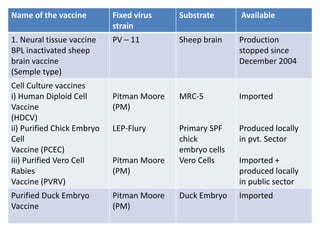
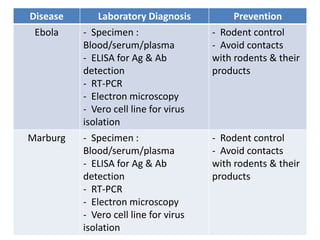
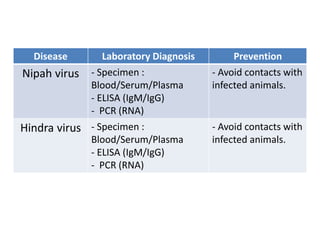
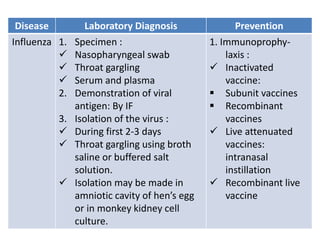
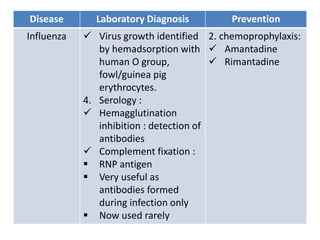
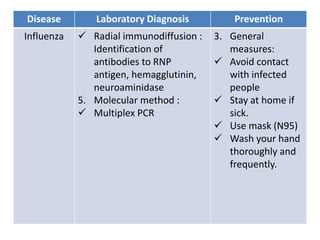
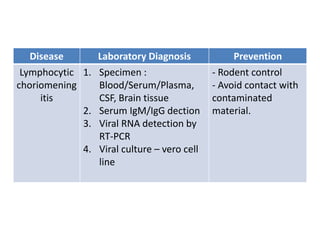
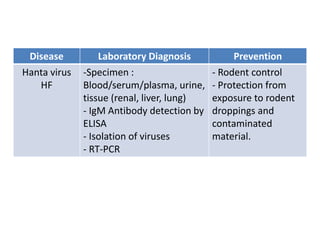
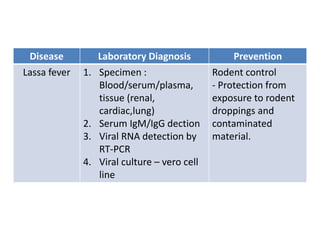
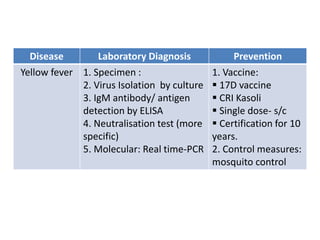
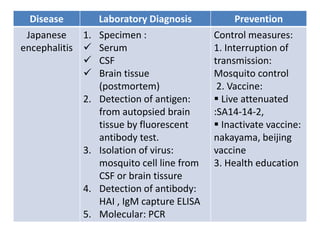
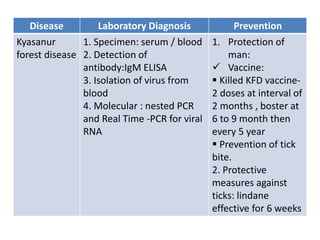
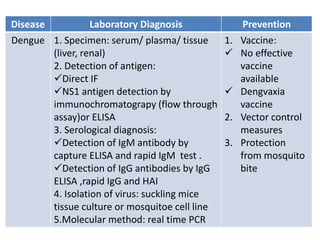
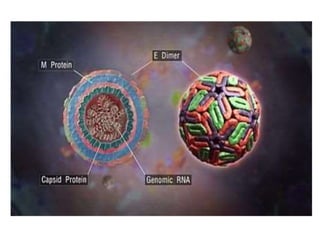
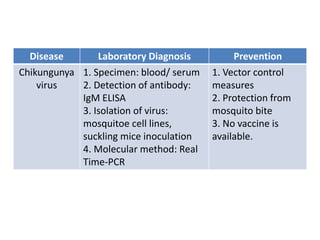
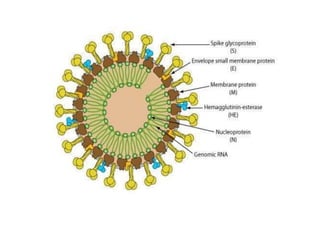
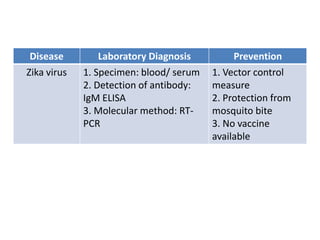
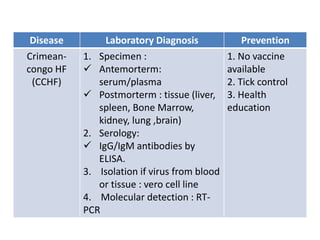
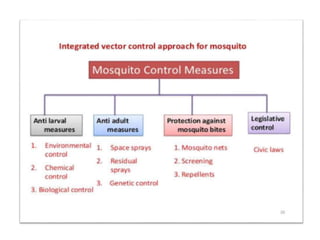
This document discusses zoonoses, which are diseases that can be transmitted between animals and humans. It covers the definition of zoonoses, factors that influence their prevalence, classification, and modes of transmission. Specific zoonotic diseases discussed include rabies, herpes B, foot and mouth disease, monkeypox, Ebola, Nipah virus, and influenza. The laboratory diagnosis and treatment of some diseases is also reviewed.