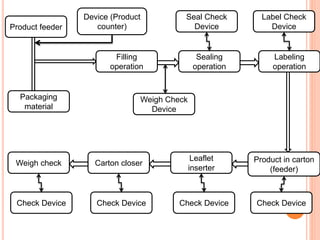
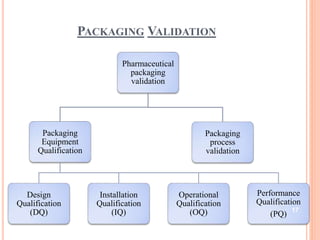
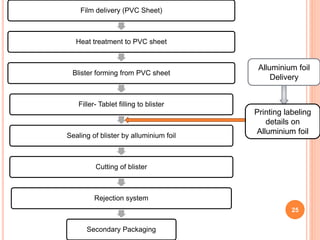
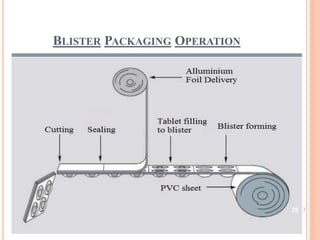
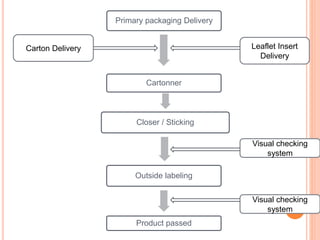
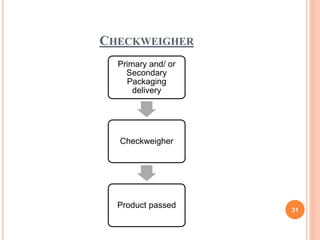
This document summarizes a seminar presentation on validation of packaging operations. It discusses the importance of packaging for pharmaceutical products and outlines key areas of packaging validation including packaging equipment qualification and packaging process validation. Specific examples of packaging processes like blister packaging and secondary packaging are described. Critical parameters and steps for these processes are identified. The presentation provides an overview of packaging validation requirements and procedures to ensure packaging processes consistently produce pharmaceutical packages that meet quality standards.
![A SEMINAR ON
VALIDATION OF
PACKAGING
OPERATIONS
Presented by: Guided by:
MR. GOMTESH MILIND DOSHI Dr. S.S CHITLANGE
M.Pharm.1st year[QAT] Sem-II Principal & Professor
Roll No. 526 Dr. D Y Patil IPSR,PIMPRI,PUNE1
Dr. D. Y. Patil Institute of Pharmaceutical Science
and Research, Pimpri, Pune](https://image.slidesharecdn.com/526gomteshpackagingvalidation-161201165427/75/Packaging-validation-1-2048.jpg)