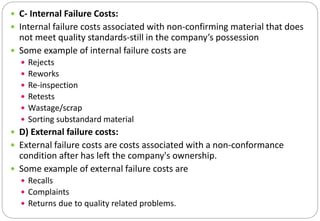
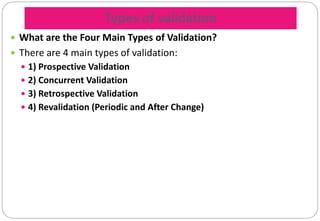
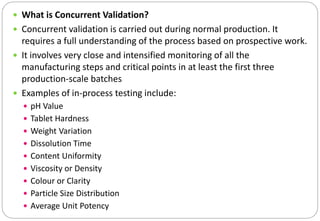
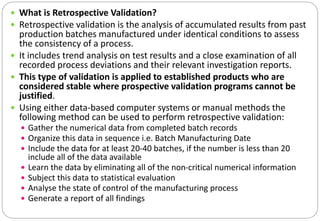
The document provides a comprehensive overview of pharmaceutical validation, detailing its importance in ensuring quality management as defined by the FDA and ISO standards. It discusses various aspects of validation including analytical test methods, equipment calibration, and the importance of training operators, while also outlining the benefits such as cost reduction, process optimization, and assurance of quality. Additionally, the document explains different types of validation—prospective, concurrent, retrospective, and revalidation—and emphasizes the necessity of a Validation Master Plan (VMP) for organizing and documenting validation efforts.