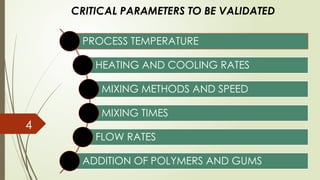
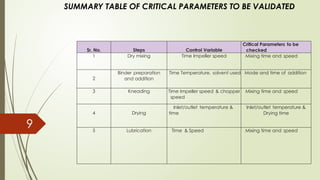
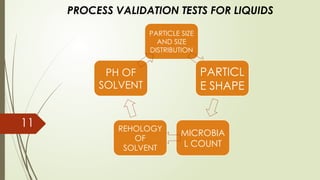
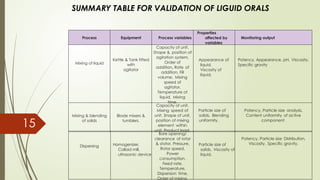
Process validation ensures that pharmaceutical products like ointments, creams, and liquid orals are consistently produced with the desired quality. It involves verifying that manufacturing processes are controlled and reproducible. Key stages include equipment qualification, process qualification, and continued process verification, ensuring safety, efficacy, and regulatory compliance.