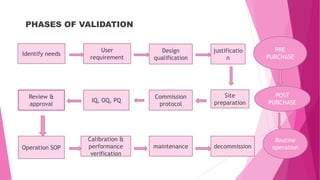
This document provides an overview of pharmaceutical validation. It defines validation as proving that processes, methods, or activities will consistently produce a quality product meeting predetermined specifications. The major types of validation discussed are process, cleaning, equipment, and analytical method validation. Process validation aims to demonstrate a process is capable of consistently producing a product meeting its quality standards. Prospective, concurrent, and retrospective validation approaches are described. Equipment validation occurs in pre-purchase, post-purchase, and routine operation phases to ensure equipment functions as intended. Validation helps ensure pharmaceutical products meet quality standards.