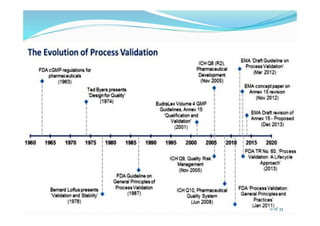
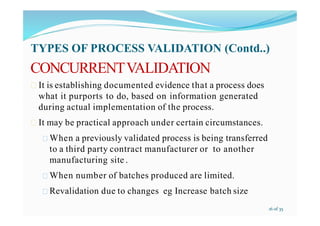
The presentation provides an overview of validation, verification, and qualification processes. It defines key terms according to FDA and WHO guidelines and provides examples. Validation ensures that processes consistently produce the expected results. Qualification proves that systems and equipment are properly installed and work correctly. Verification establishes the truth or reality of something. The presentation outlines types of validation and qualification processes and discusses reasons for validation in the pharmaceutical industry.



![DEFINITIONS (As per FDA)
Validation means confirmation by examination and
provision of objective evidence that the particular
requirements for a specific intended use can be
consistently fulfilled. [CFR 21 Part 820.3(z)]
Verification means confirmation by examination and
provision of objective evidence that specified
requirements have been fulfilled. [CFR 21 Part 820.3(aa)]
Qualification – NOT DEFINED
4 of 35](https://image.slidesharecdn.com/validation-190902110639/85/Validation-4-320.jpg)