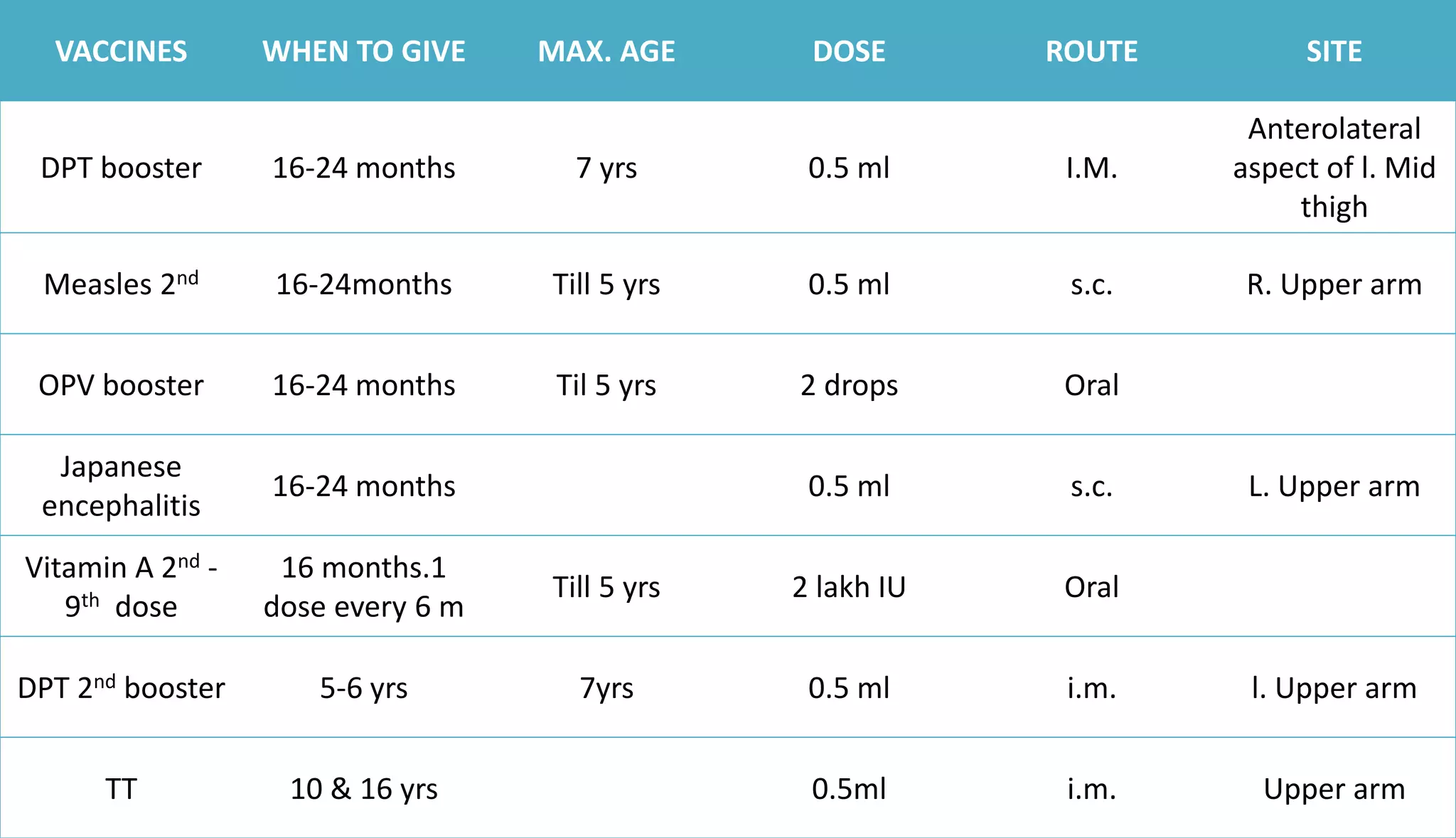
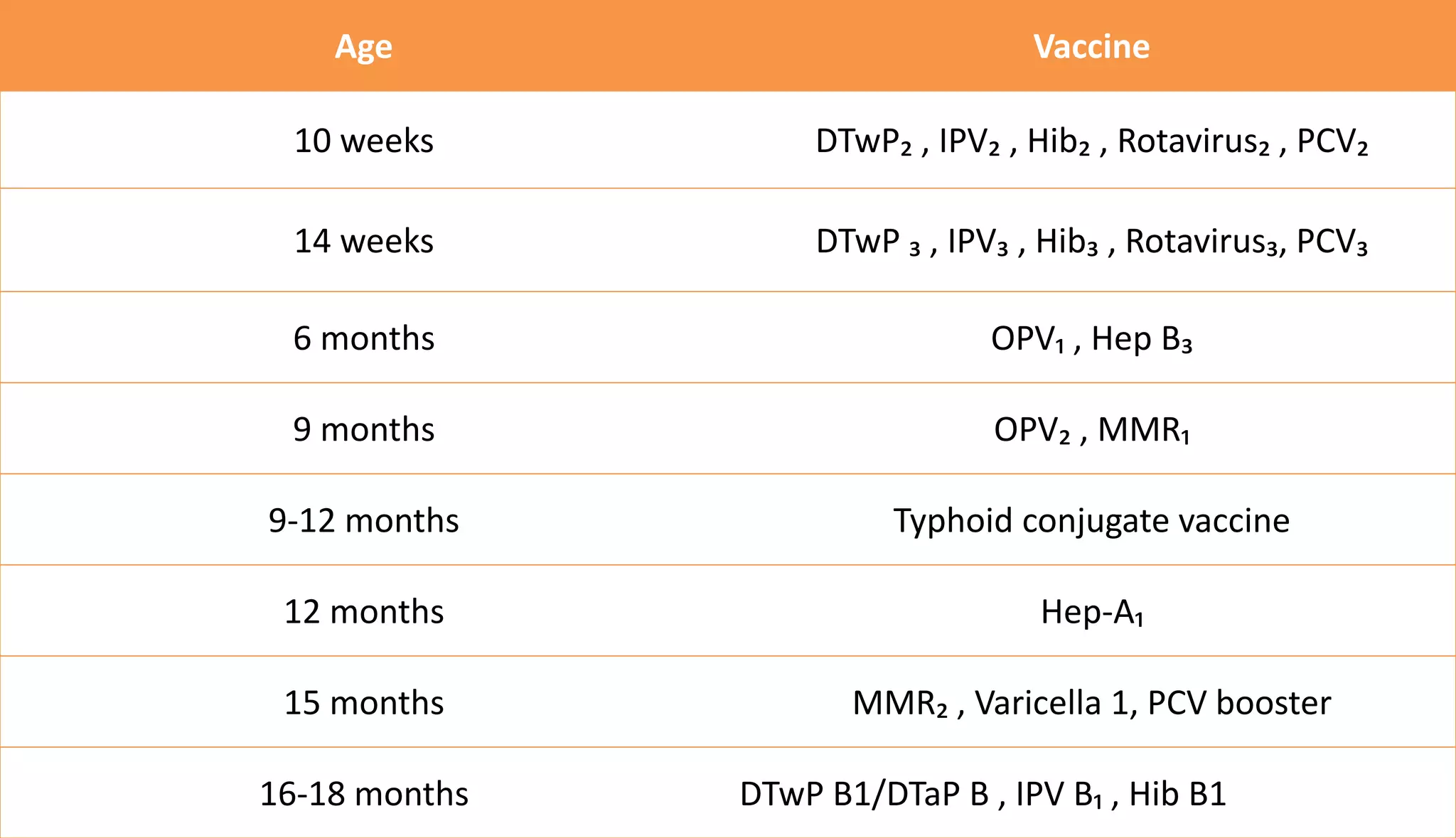
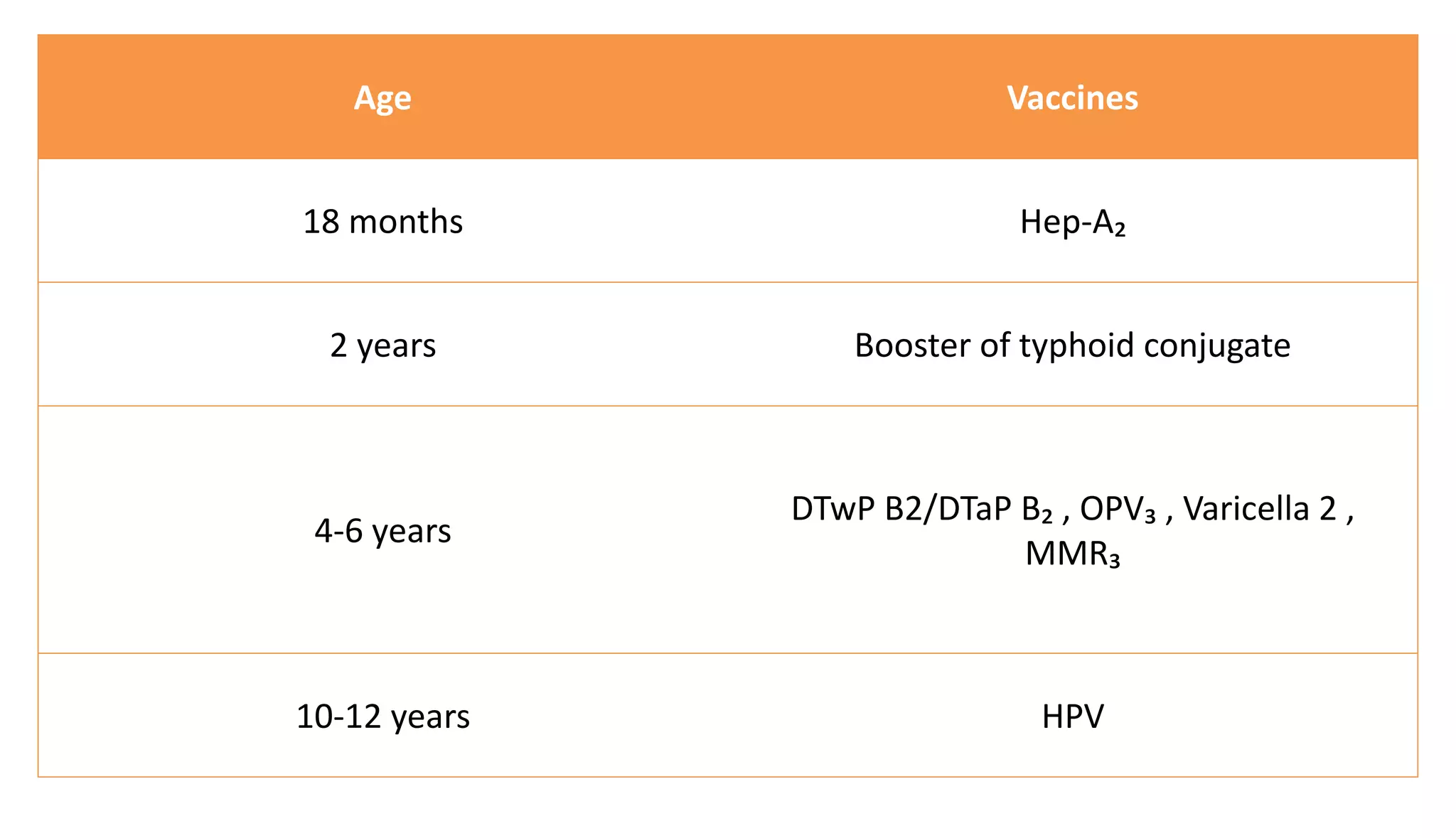
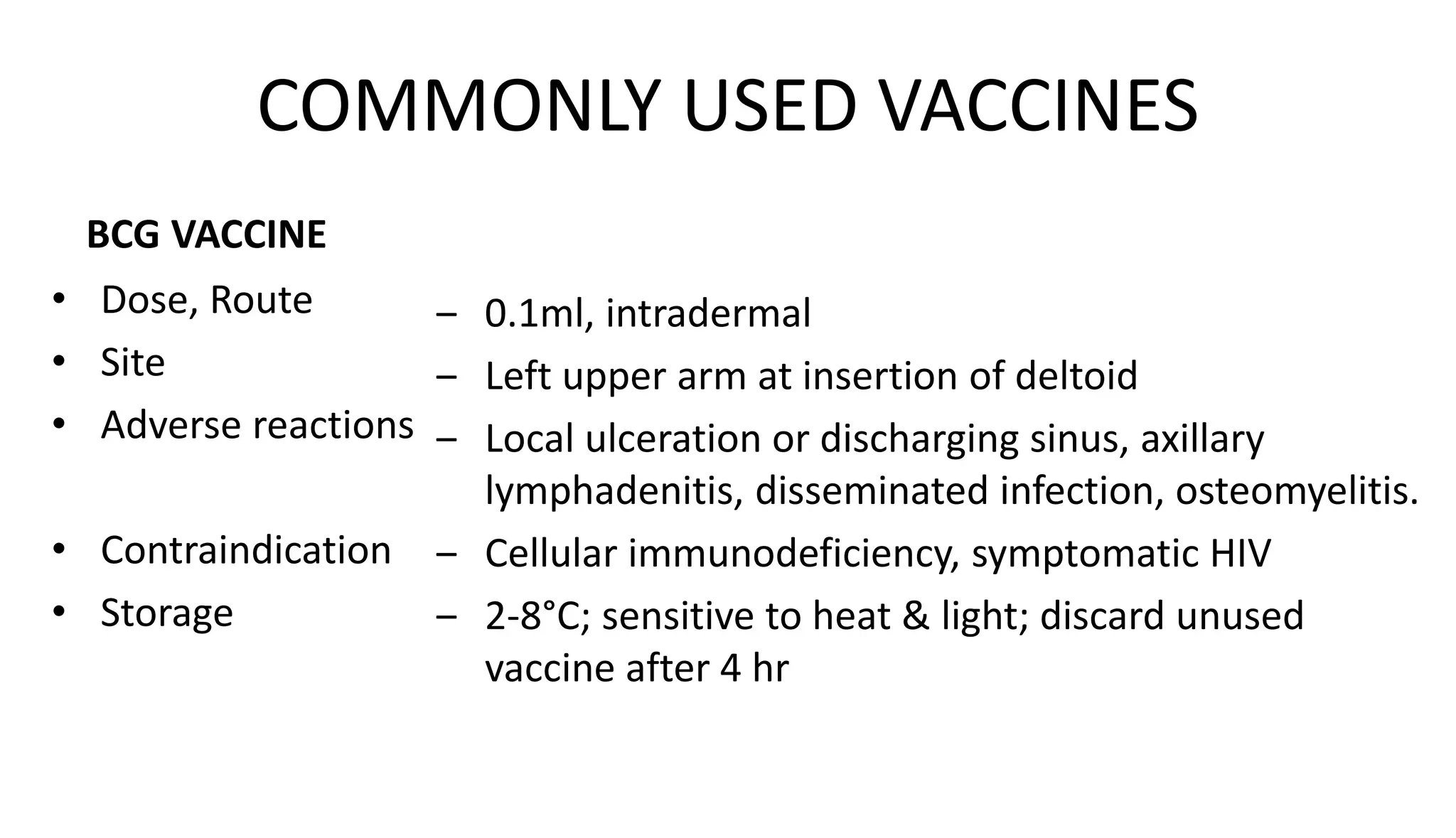
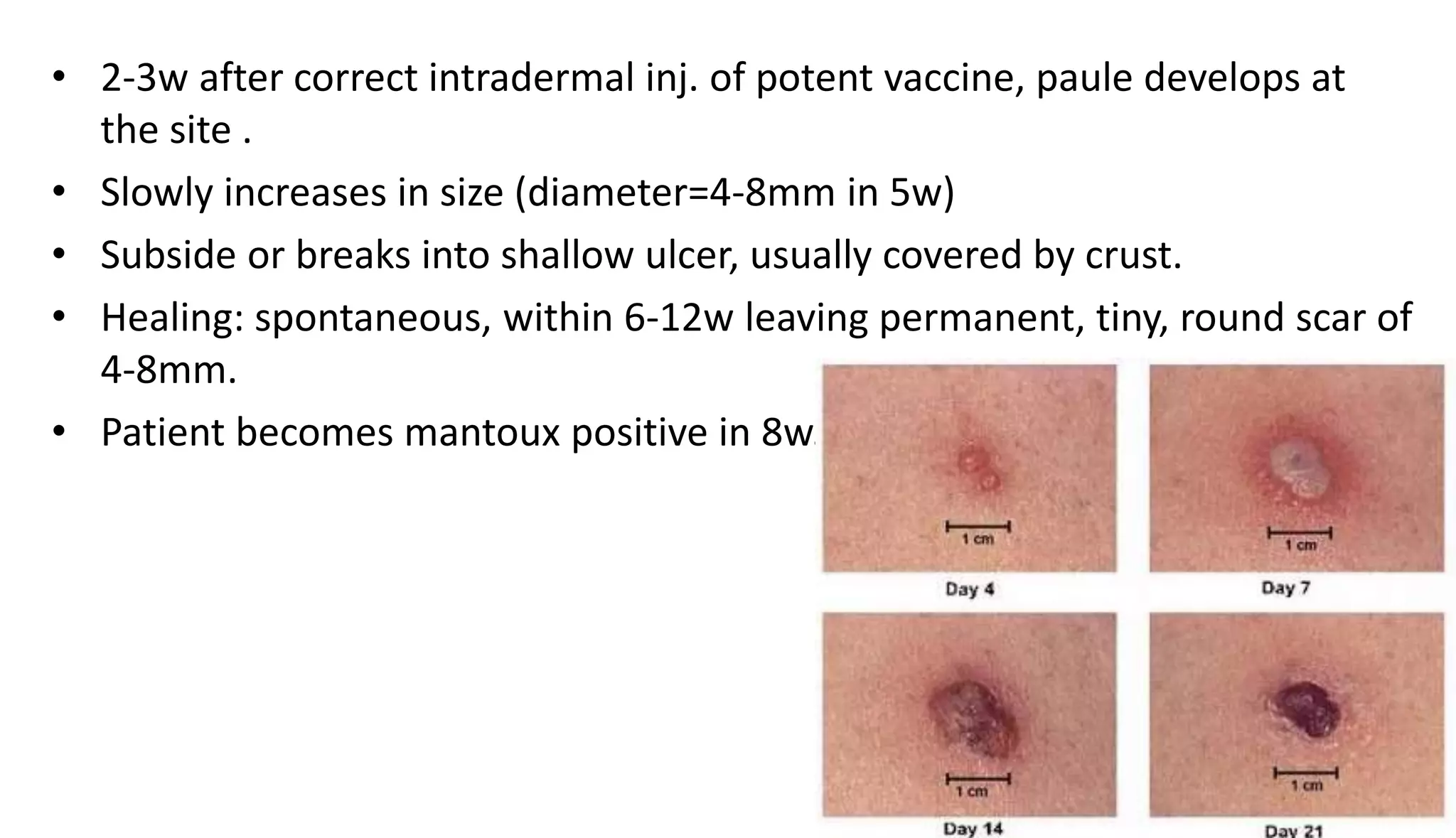
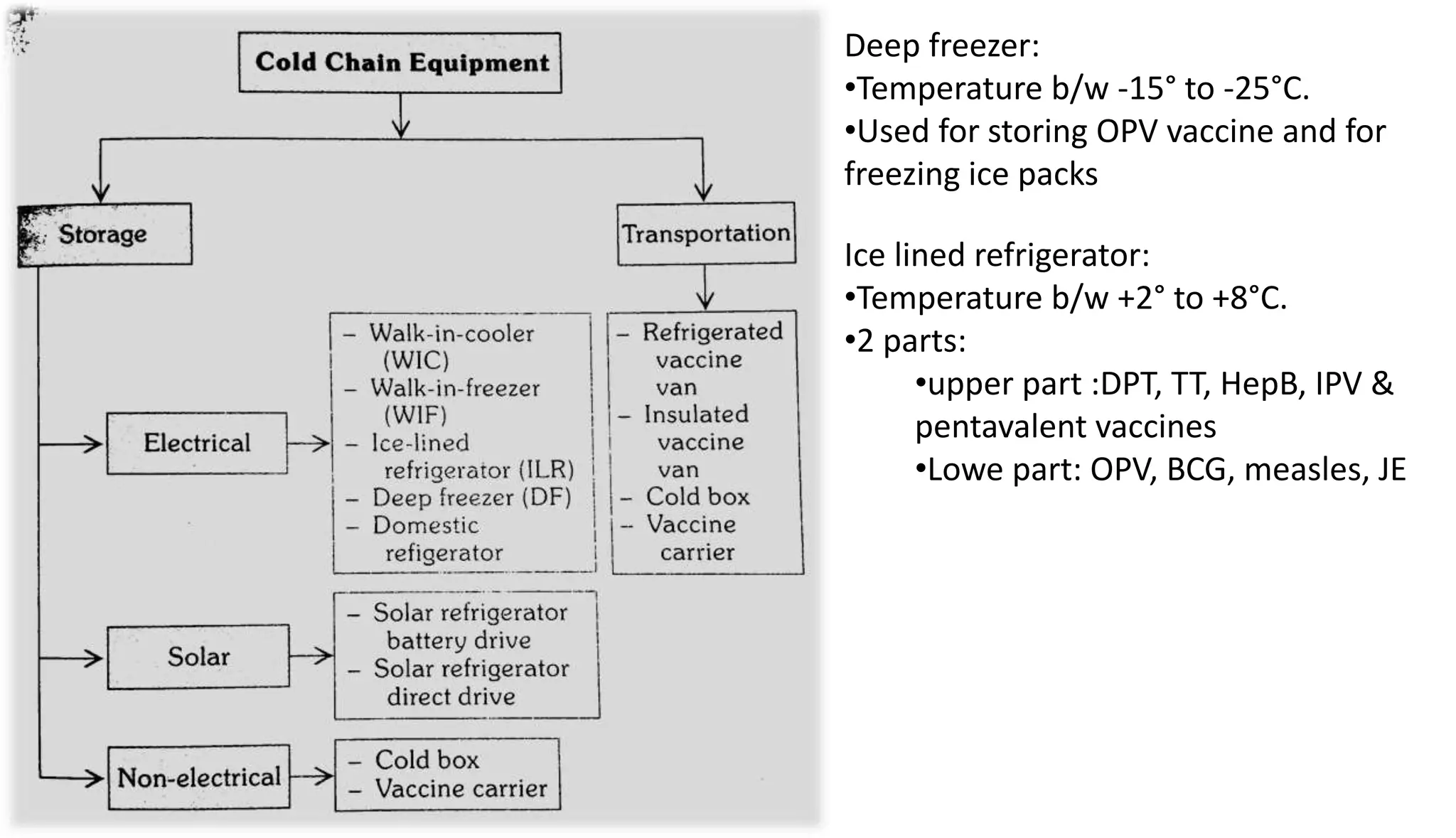
This document provides information on the national immunization schedules for infants and children in India. It includes the vaccines recommended, the ages at which they should be administered, dosage, route of administration, maximum age limits, and storage requirements. Key details provided for individual vaccines include dose, administration route and site, adverse reactions, contraindications, schedules, and cold chain storage temperatures. The schedules outline the vaccines recommended by the national program as well as by the Indian Academy of Pediatrics.