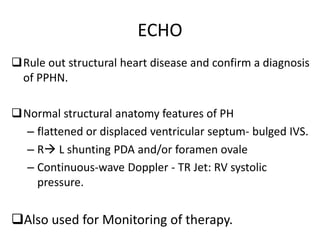
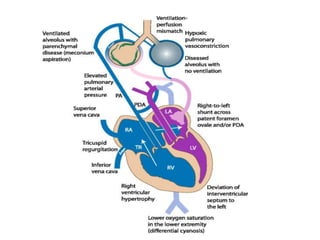
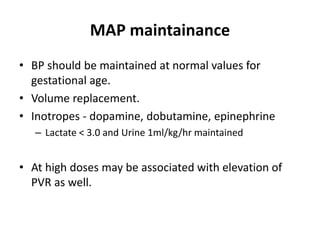
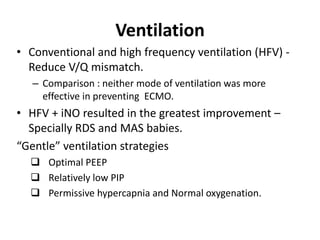
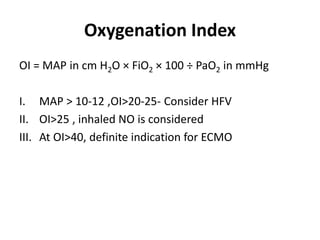
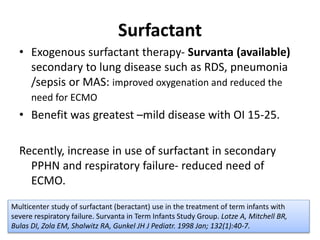
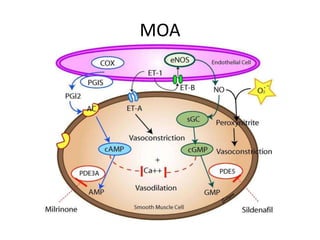
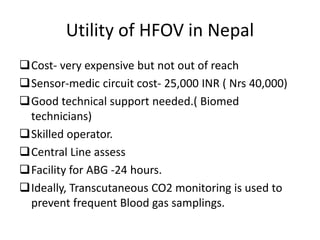
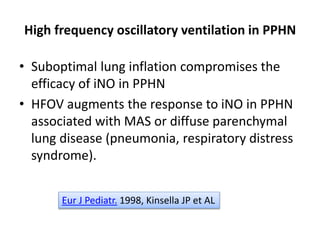
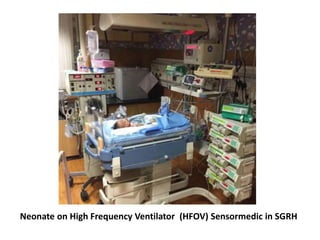
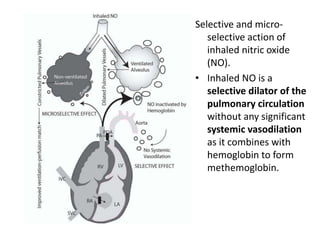
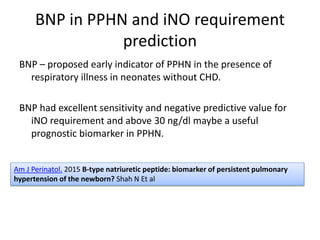
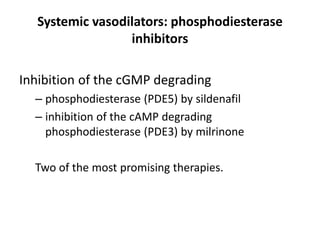
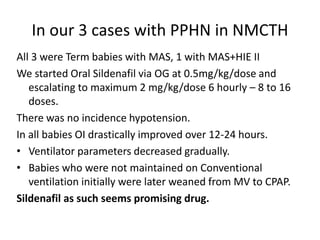
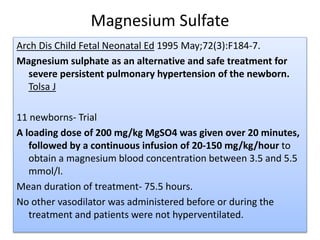
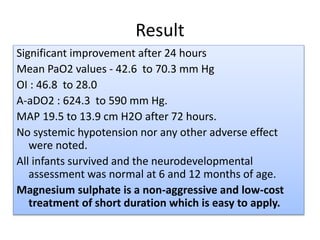
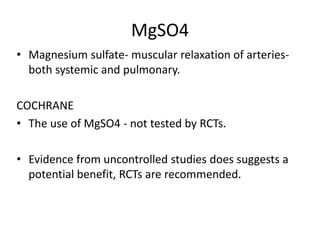
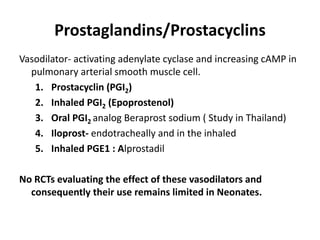
This document discusses persistent pulmonary hypertension of the newborn (PPHN) with a focus on management in resource-limited settings. It provides background on PPHN, including associated conditions, signs and symptoms, diagnostic testing, and supportive care strategies. Key interventions discussed include inhaled nitric oxide (iNO), high frequency ventilation (HFV), and sildenafil. While iNO and HFV are standard treatments, their high costs limit use in many resource-poor areas. The document explores using less expensive options like sildenafil and discusses how HFV could potentially be utilized more in Nepal with appropriate equipment, training, and support.