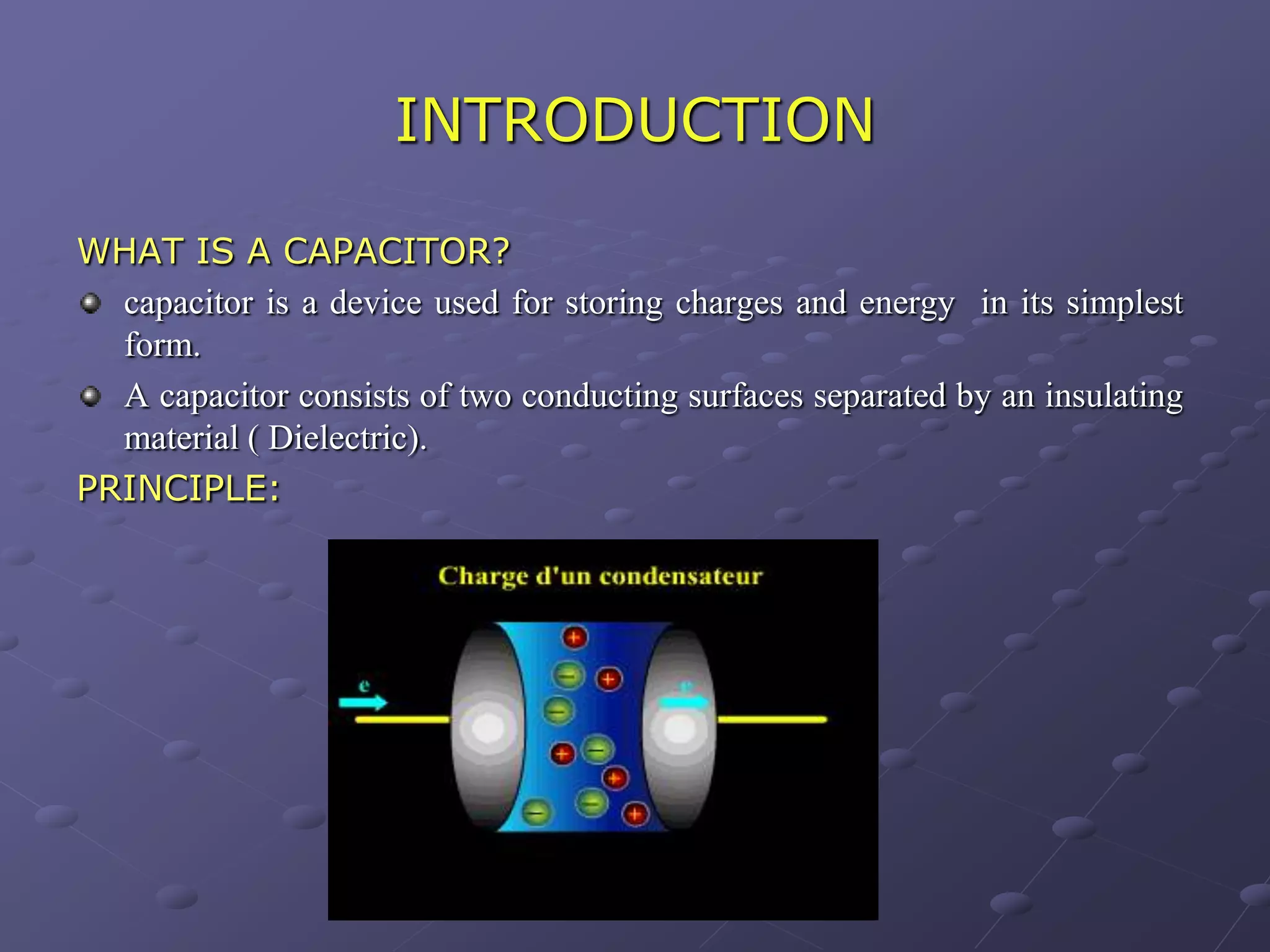
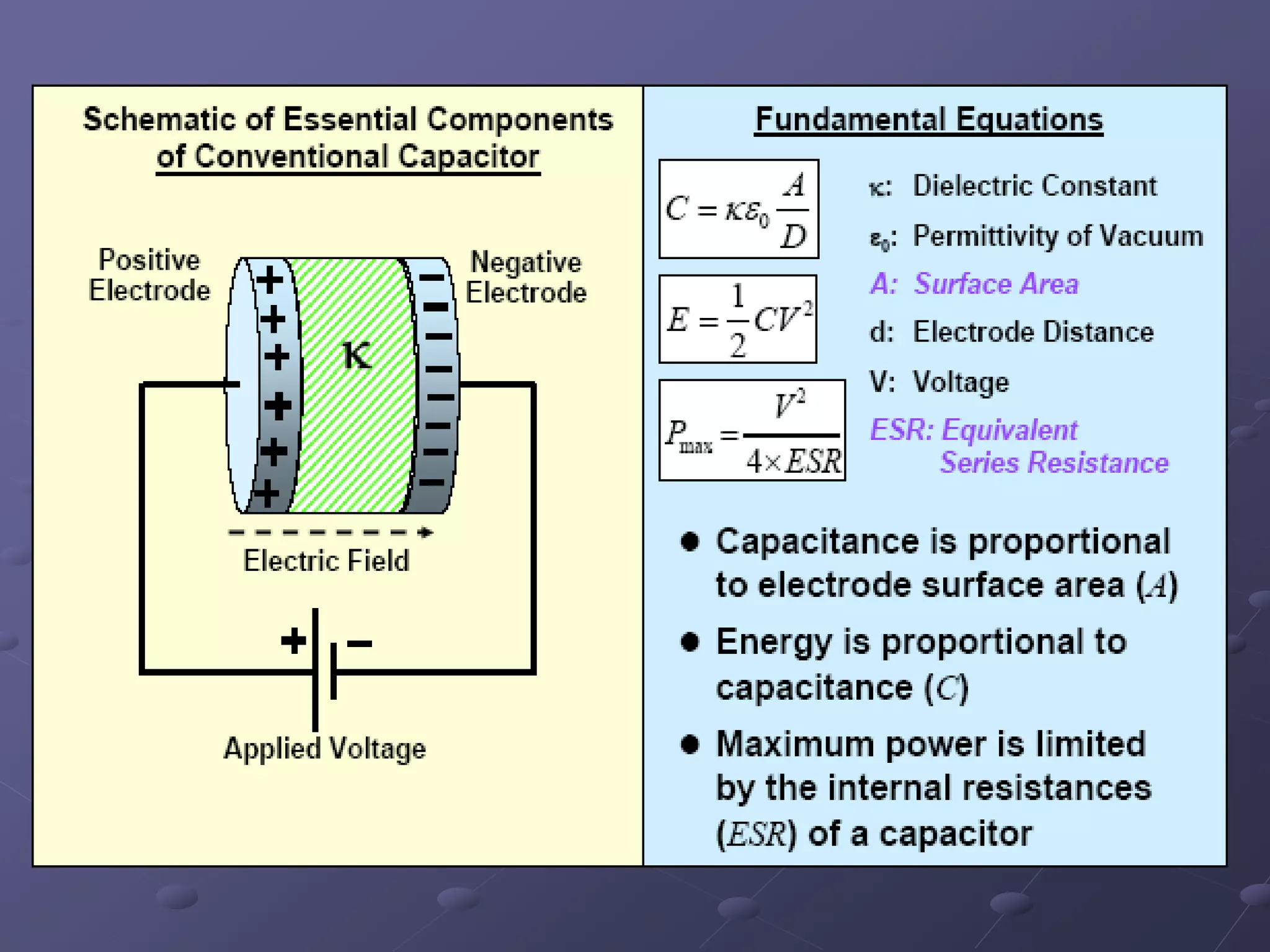
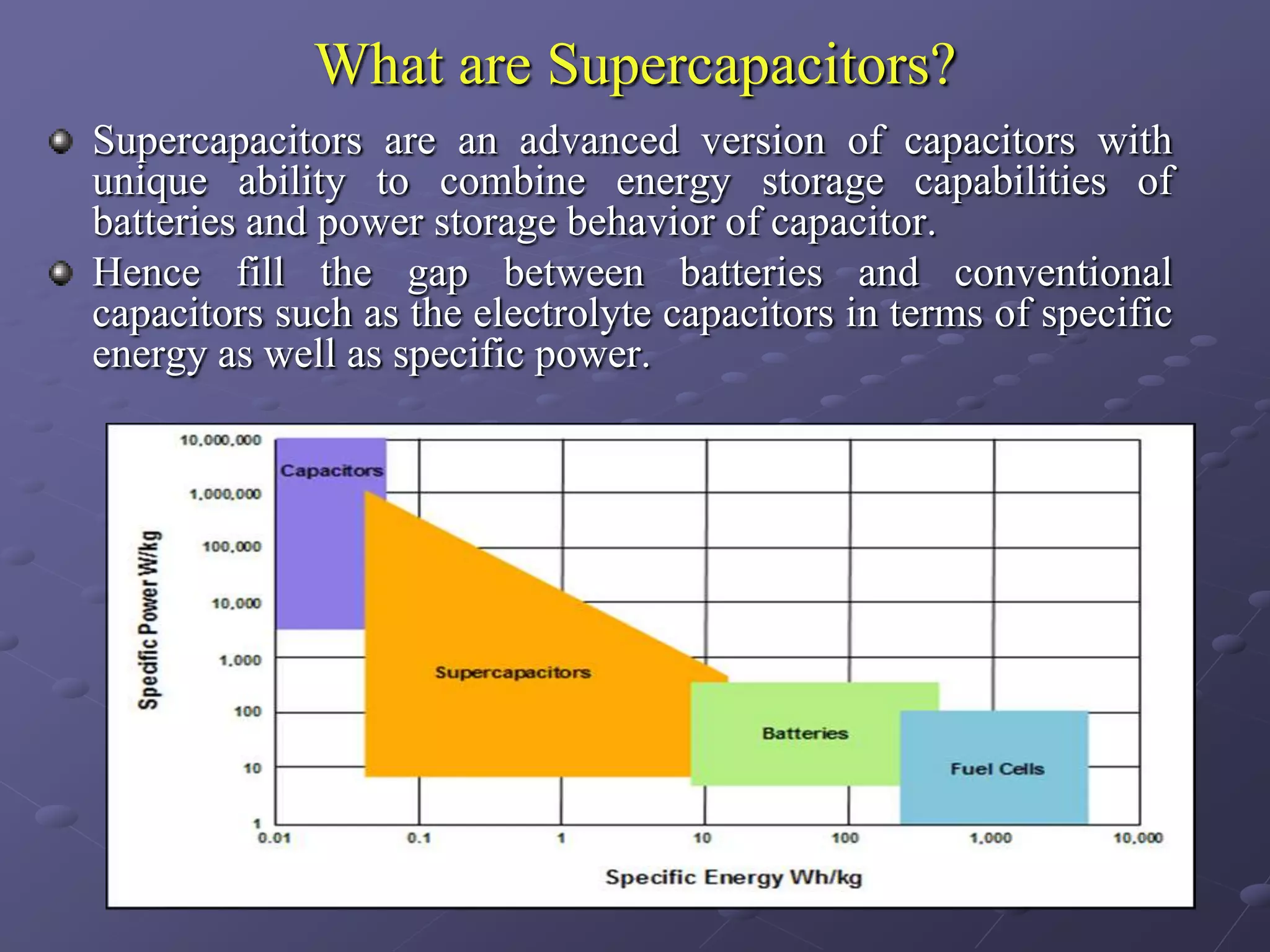
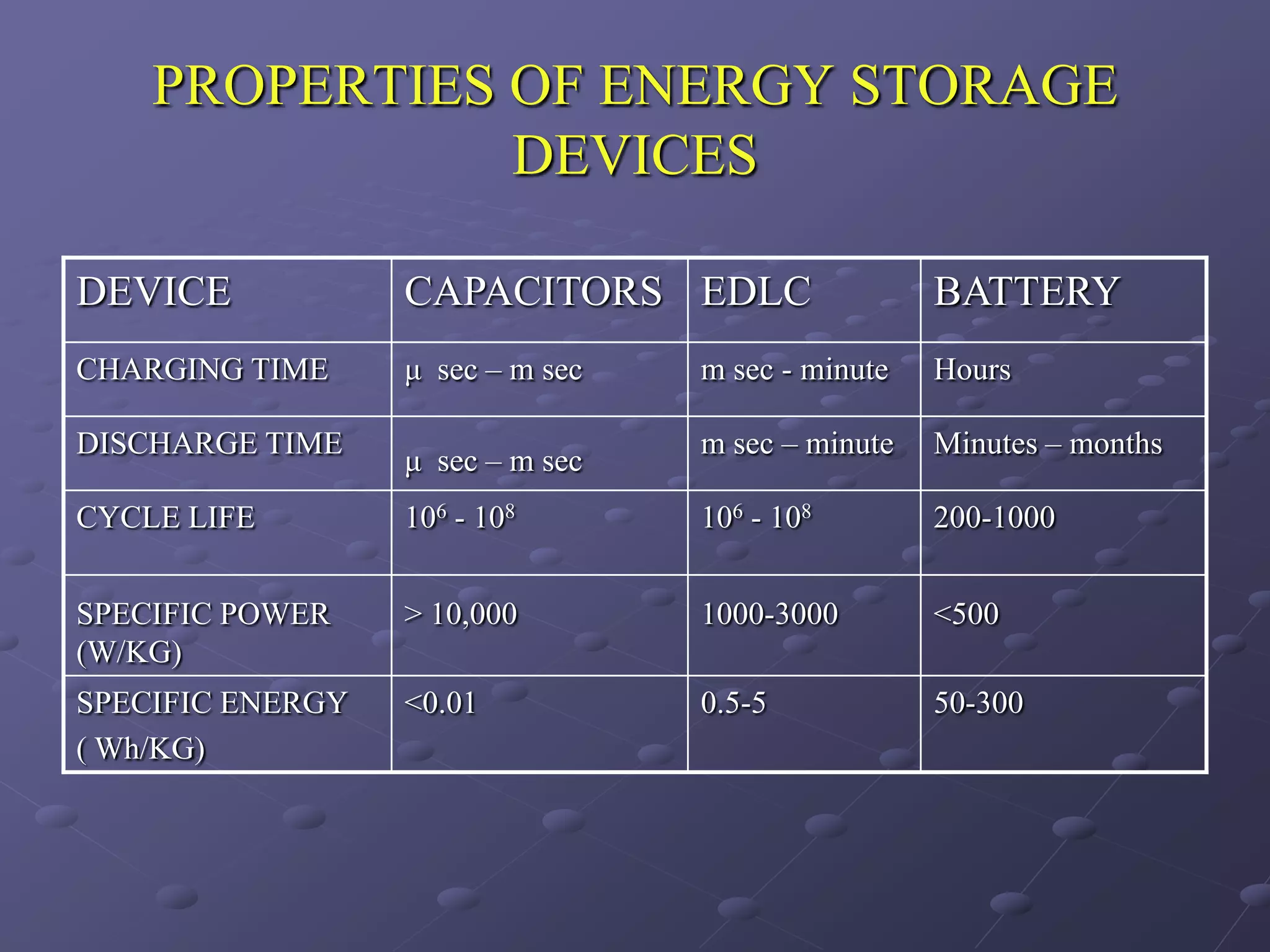
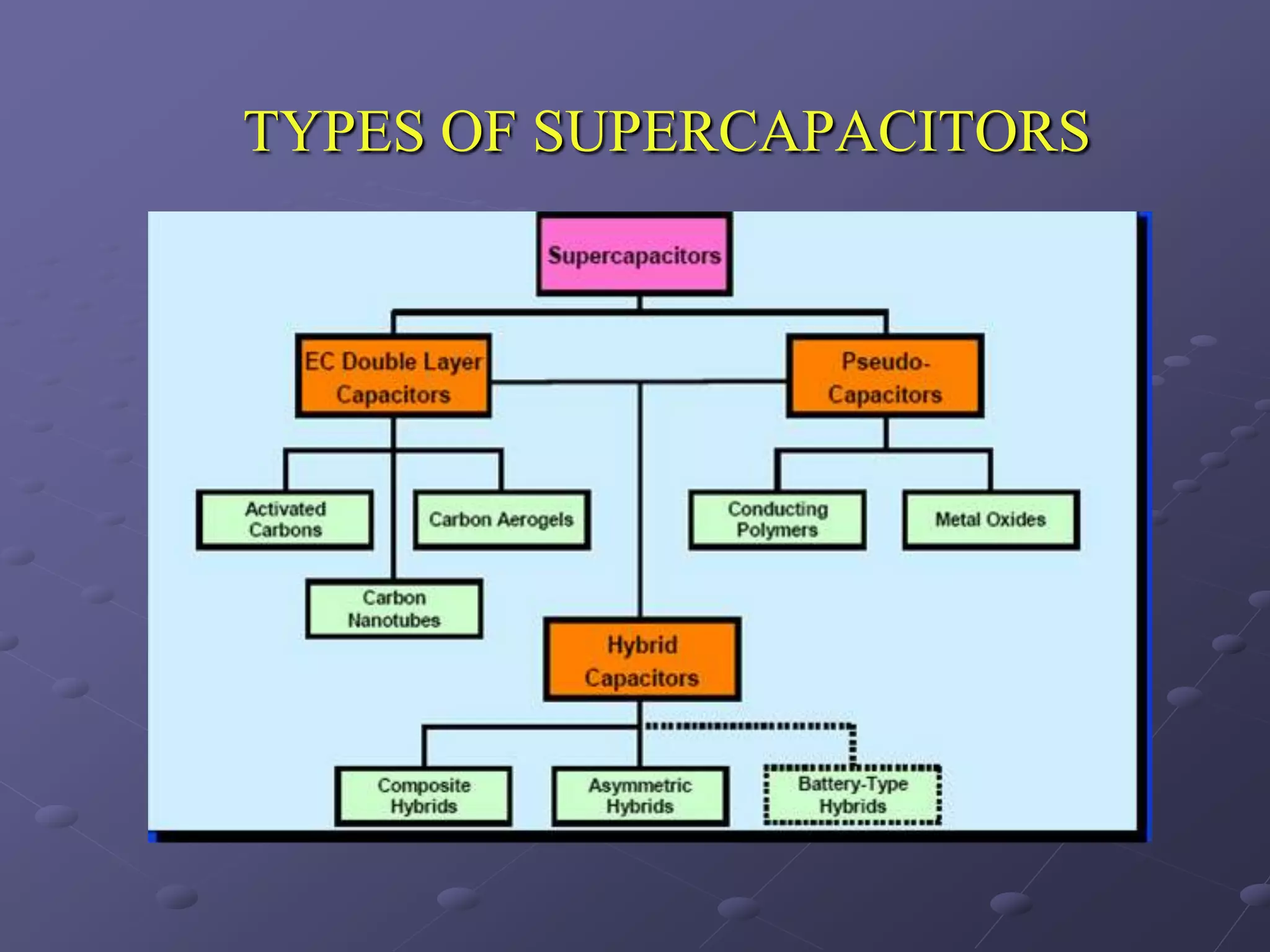
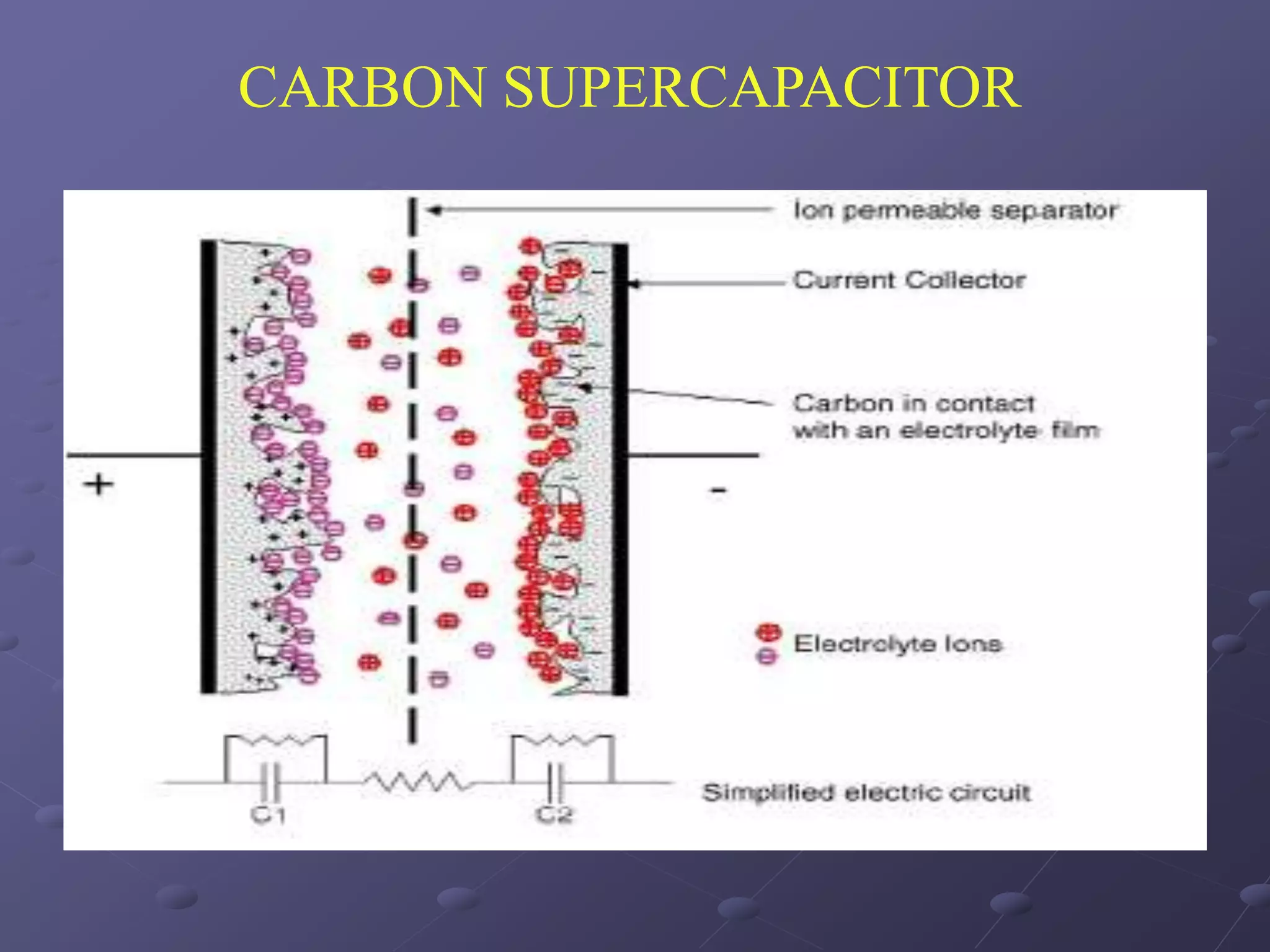
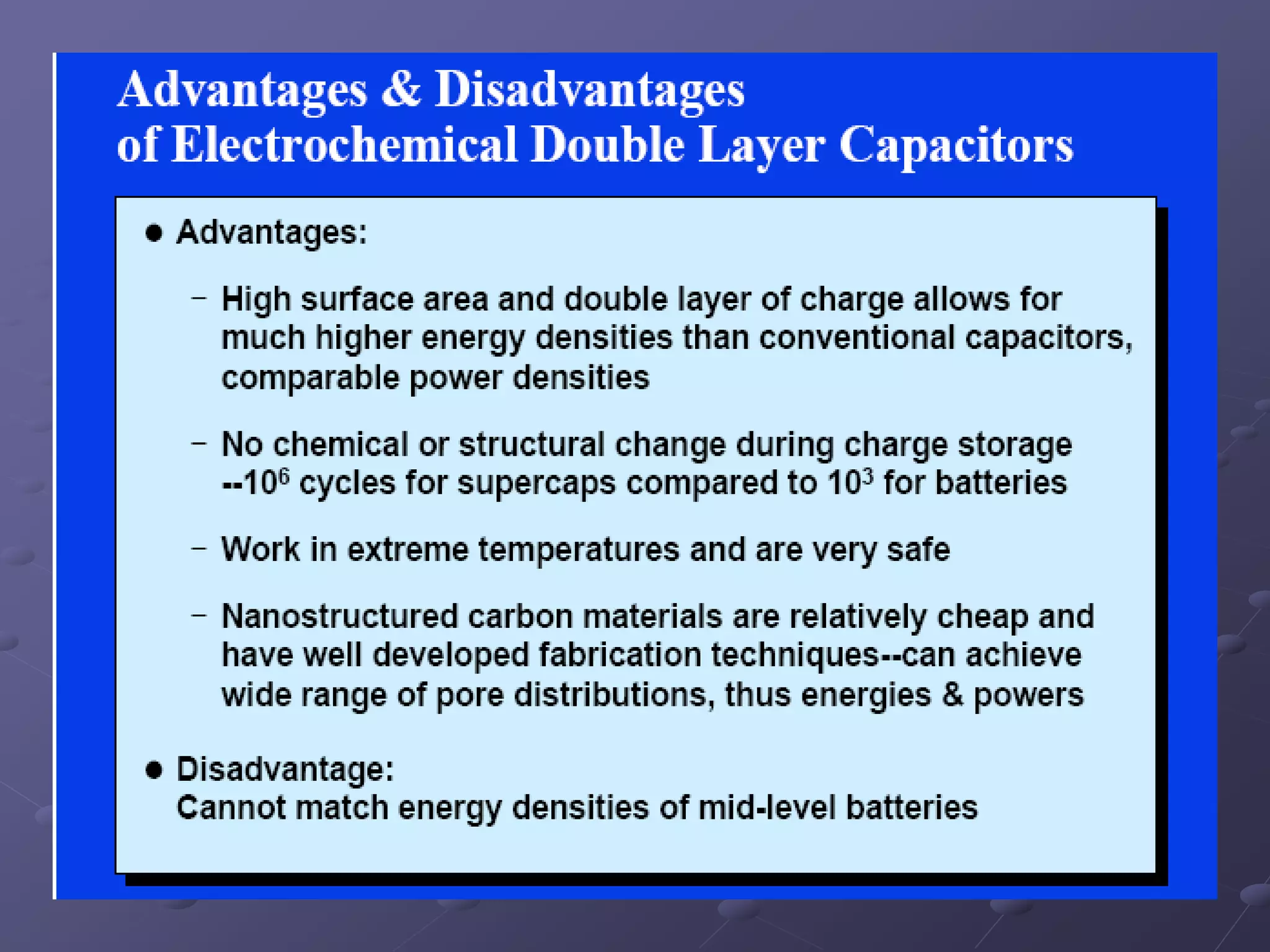
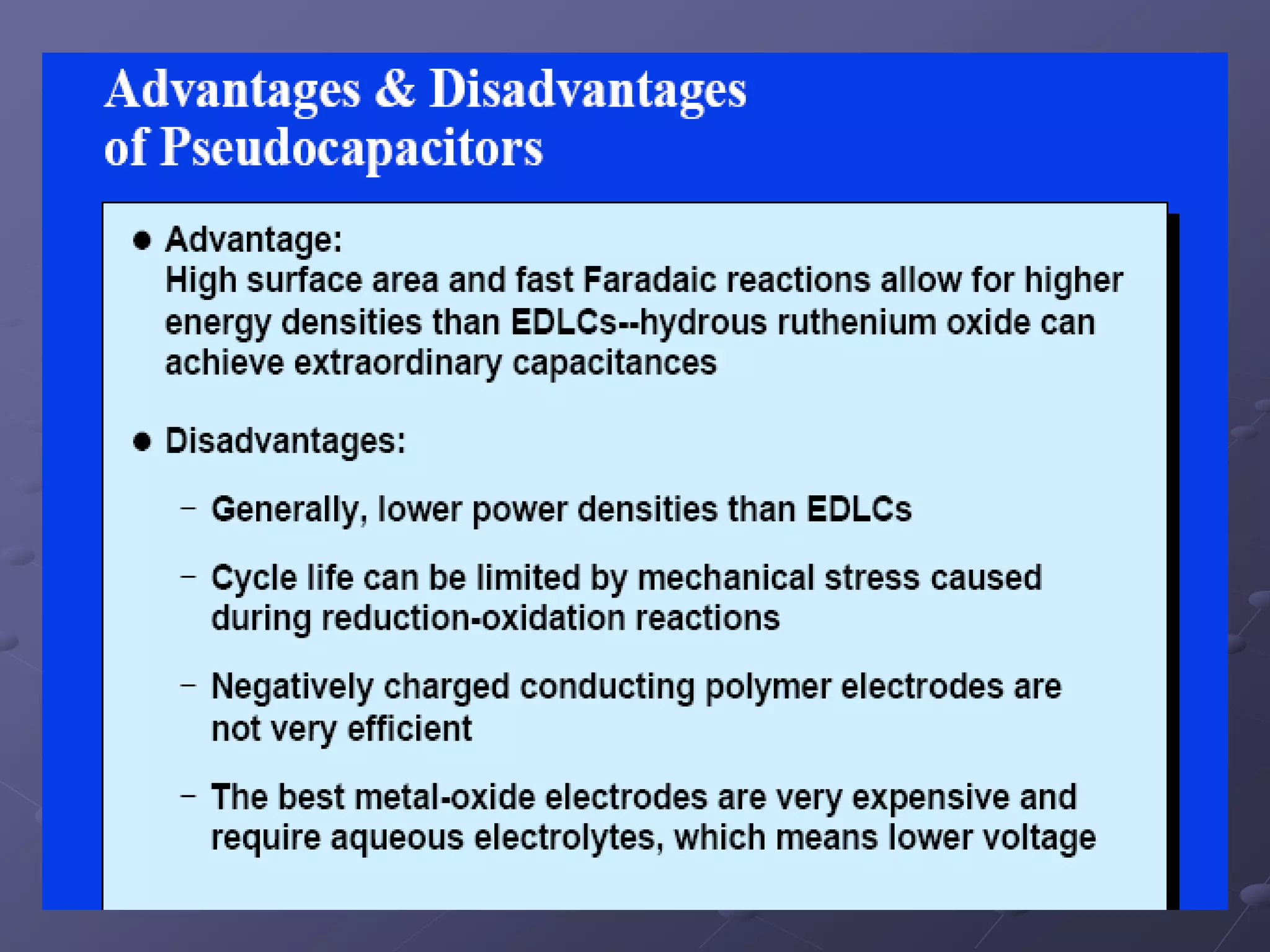
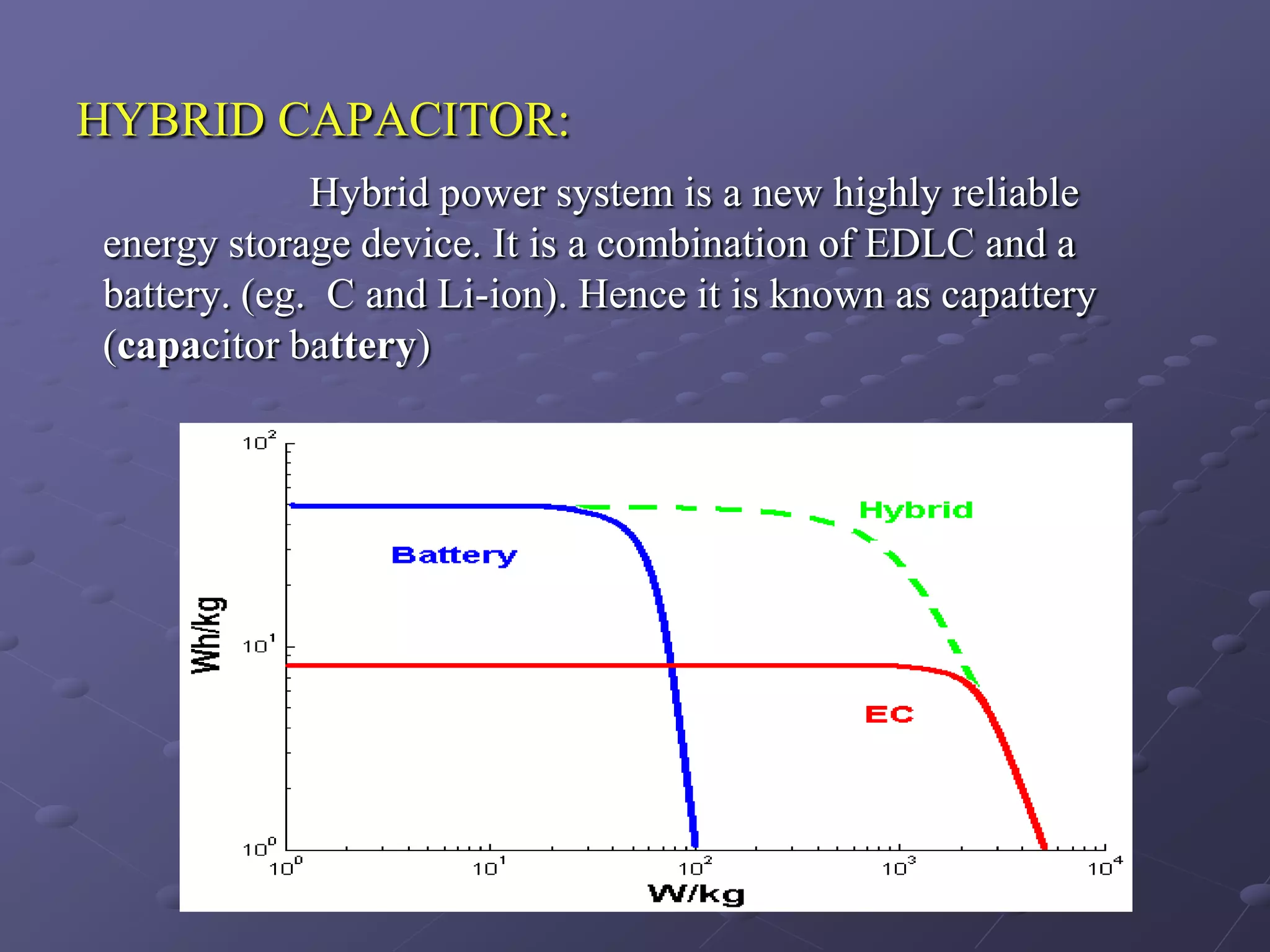
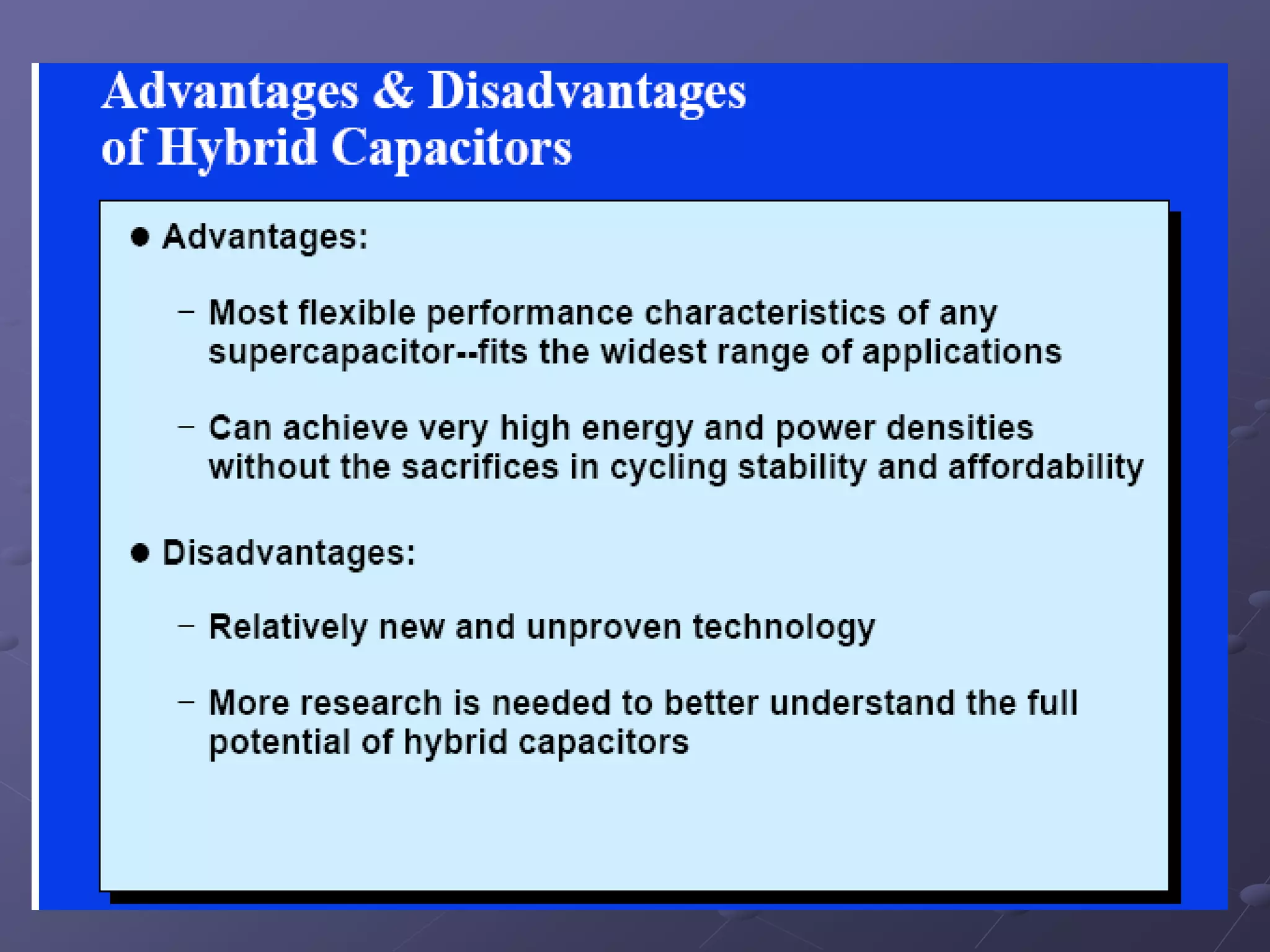
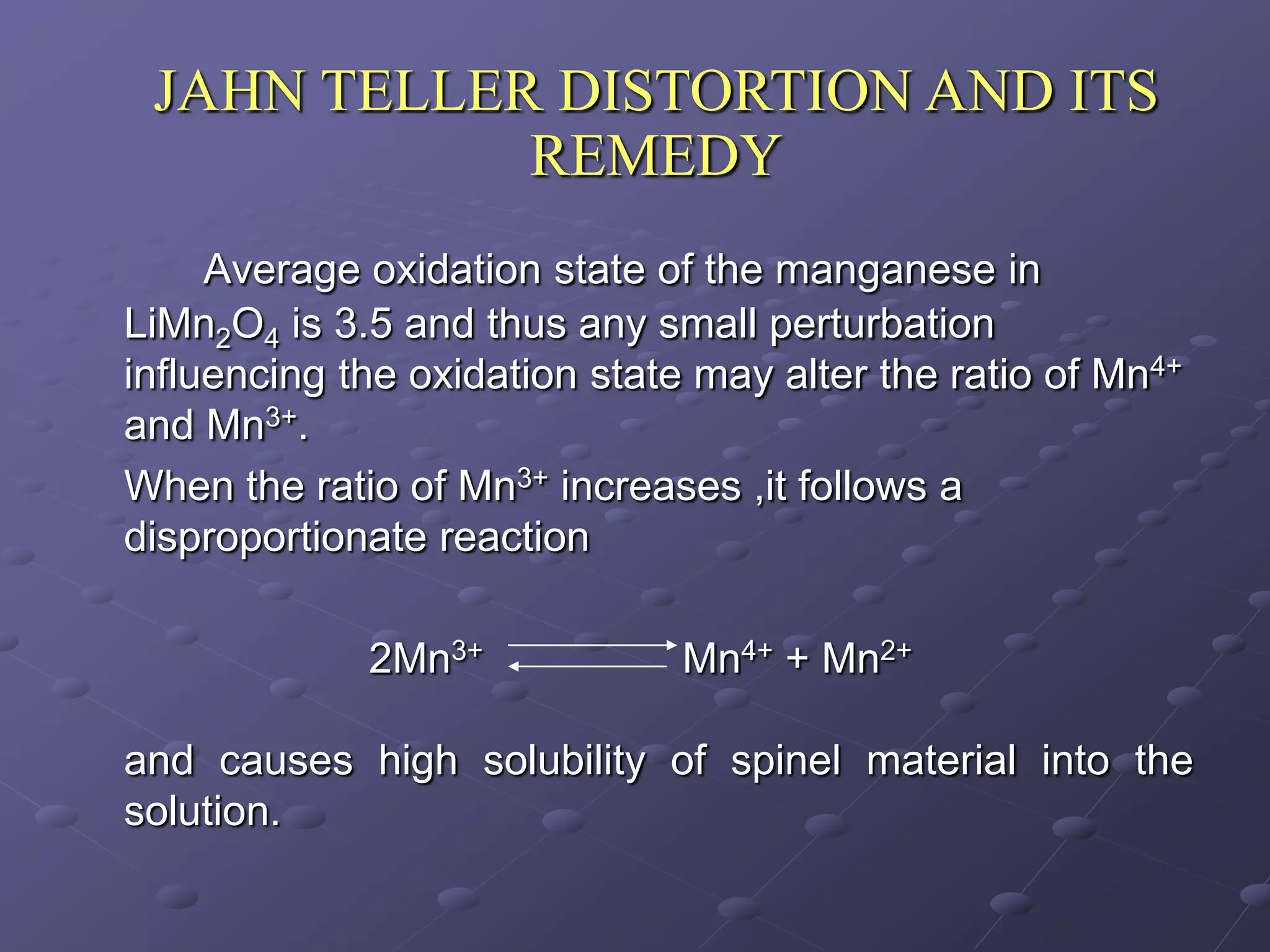
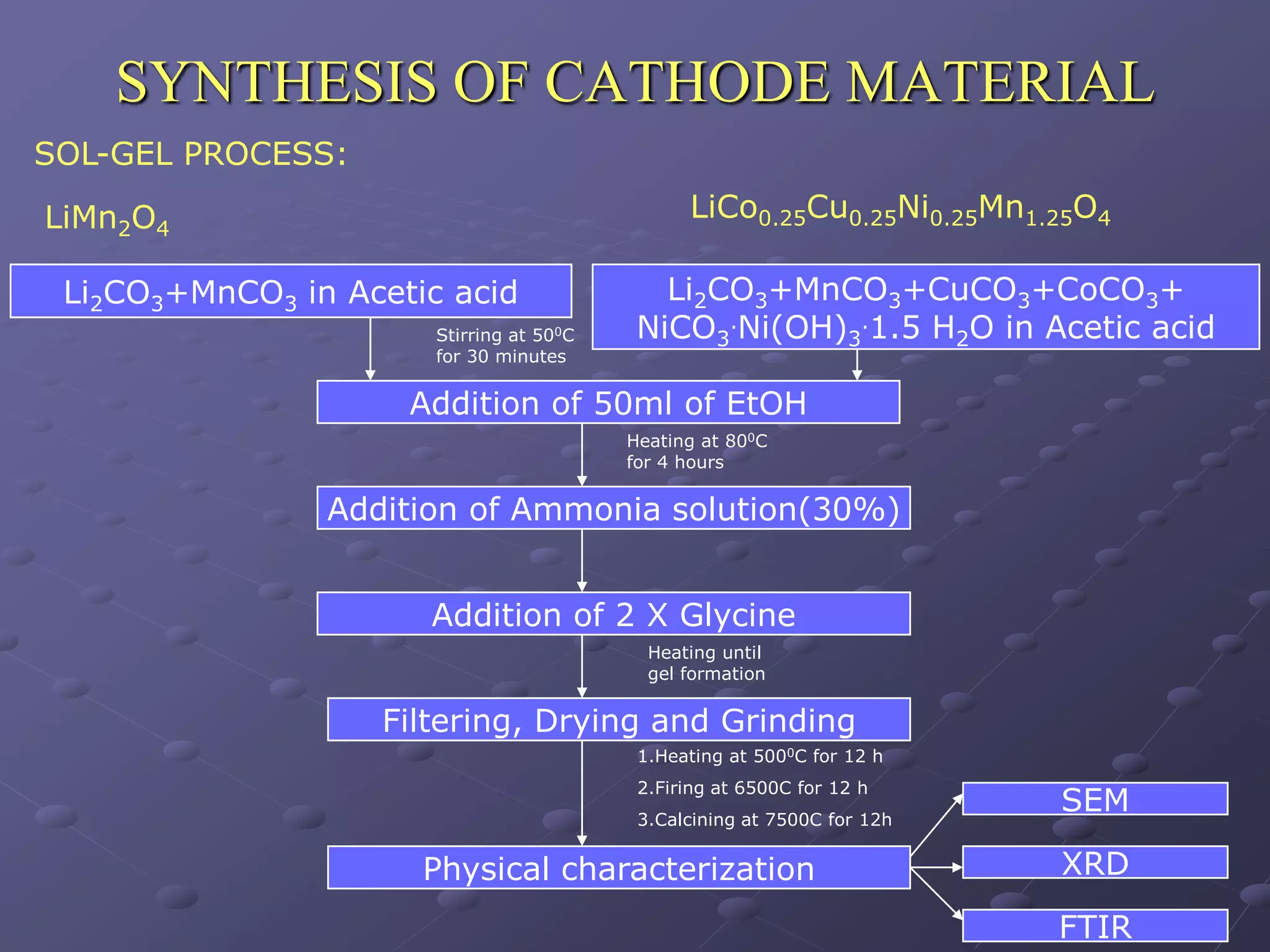
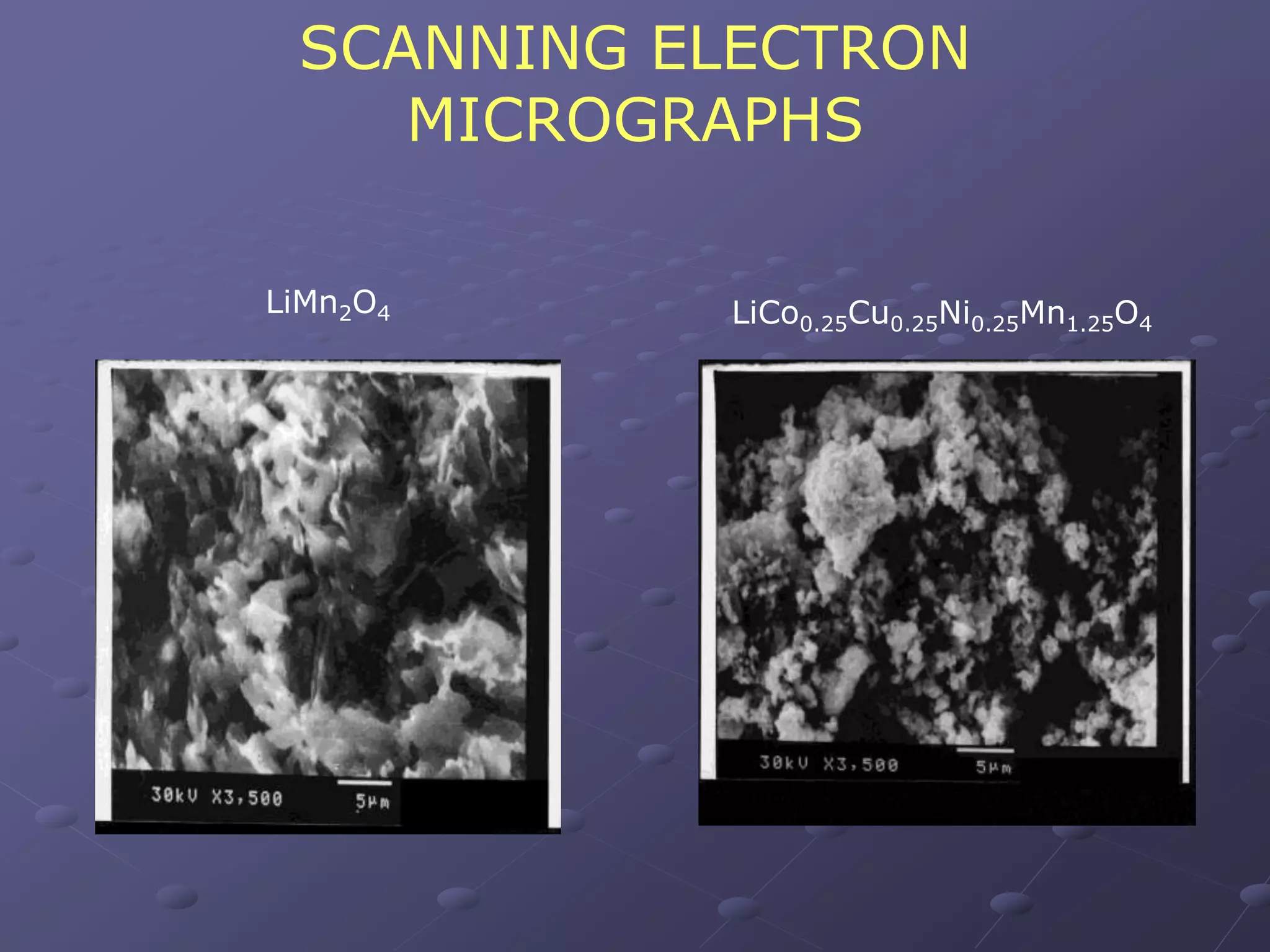
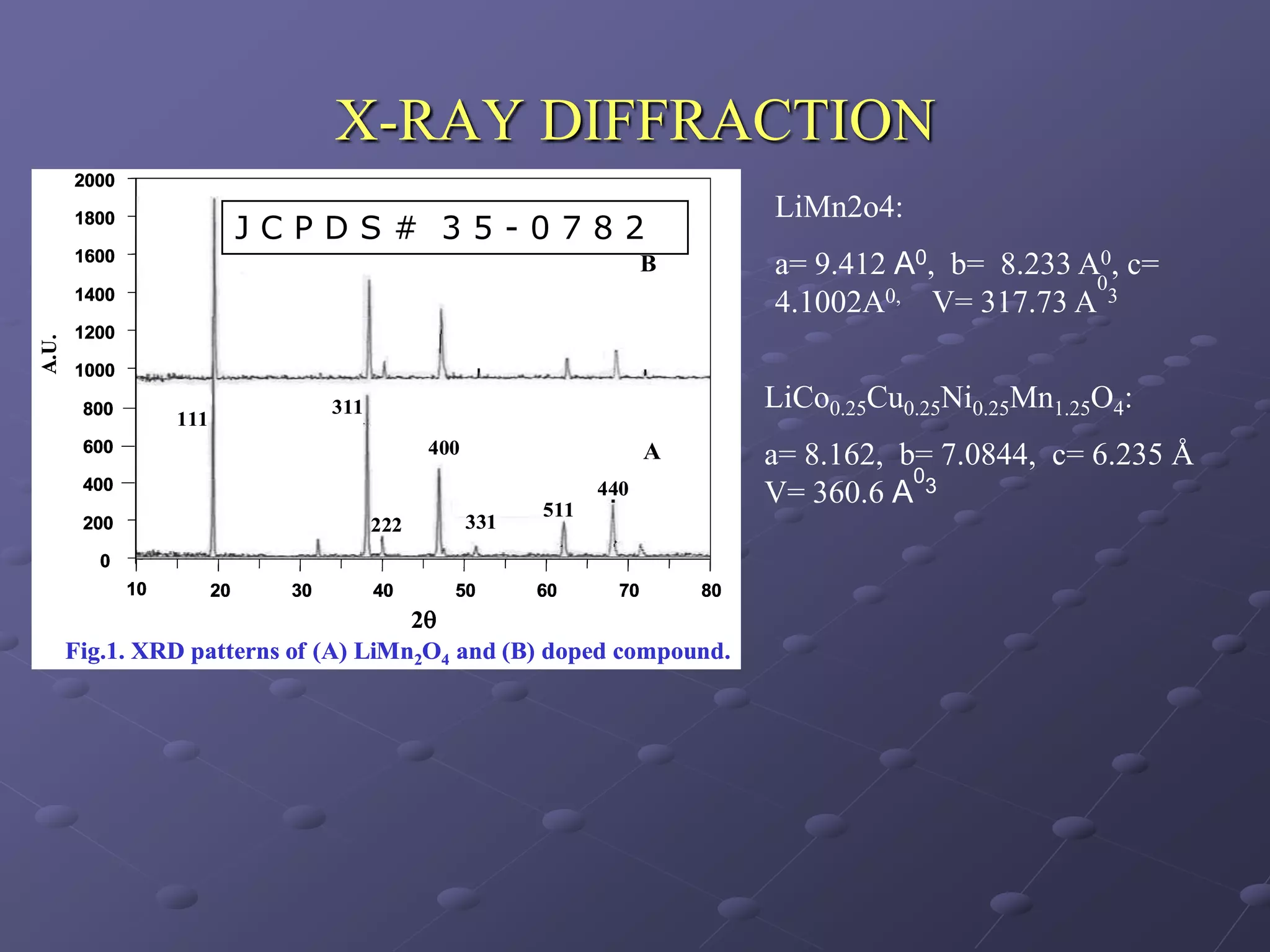
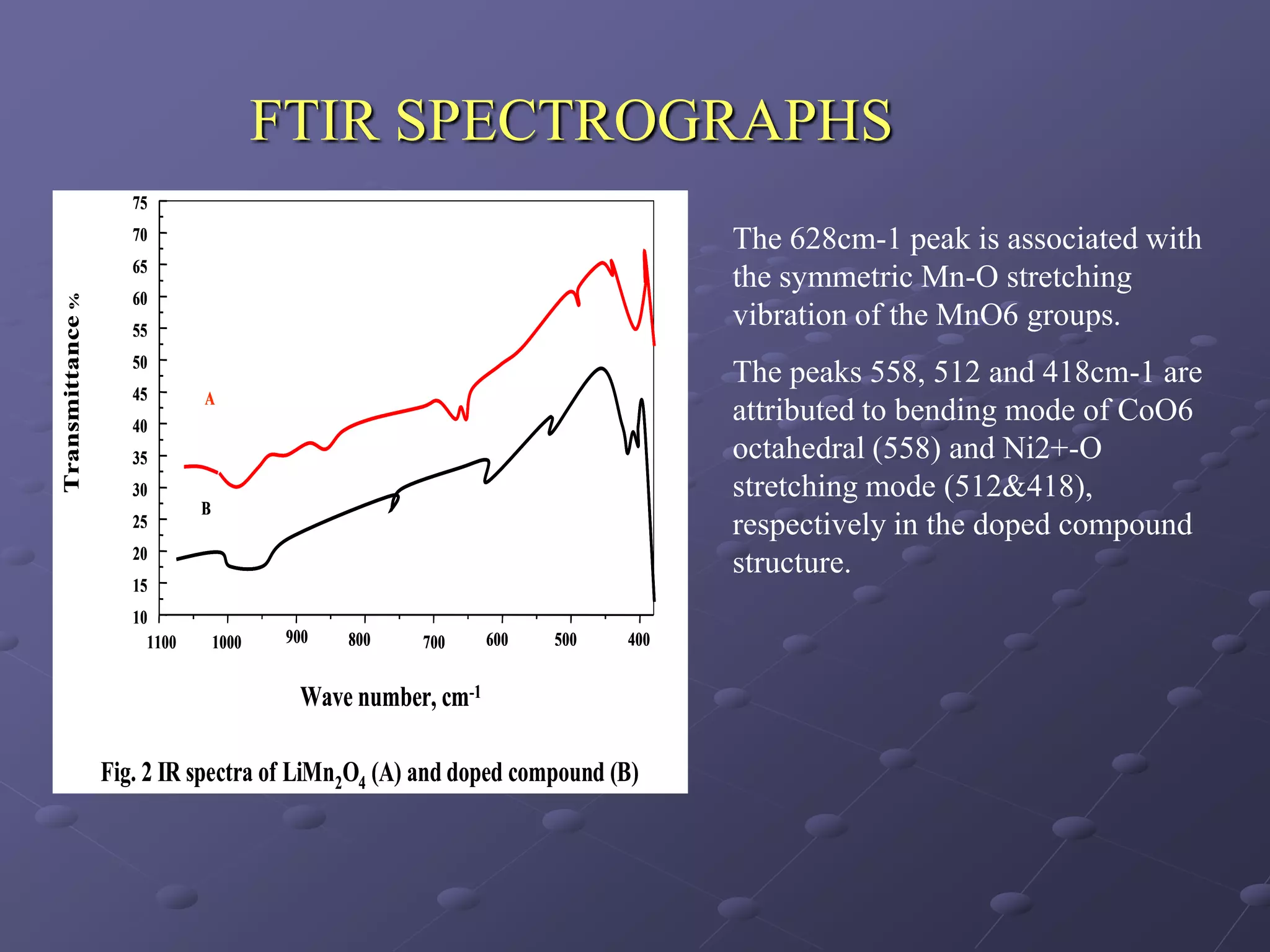
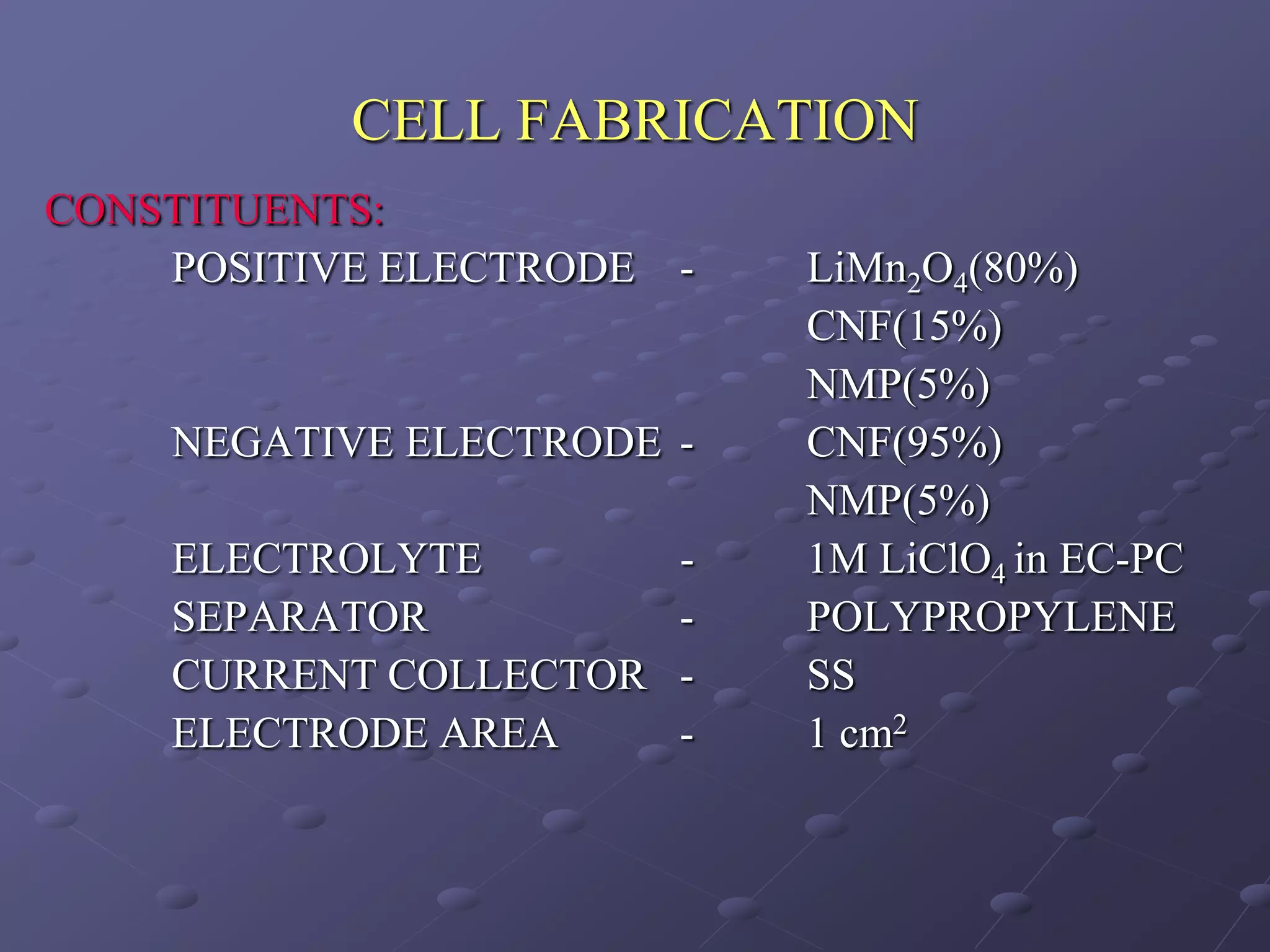
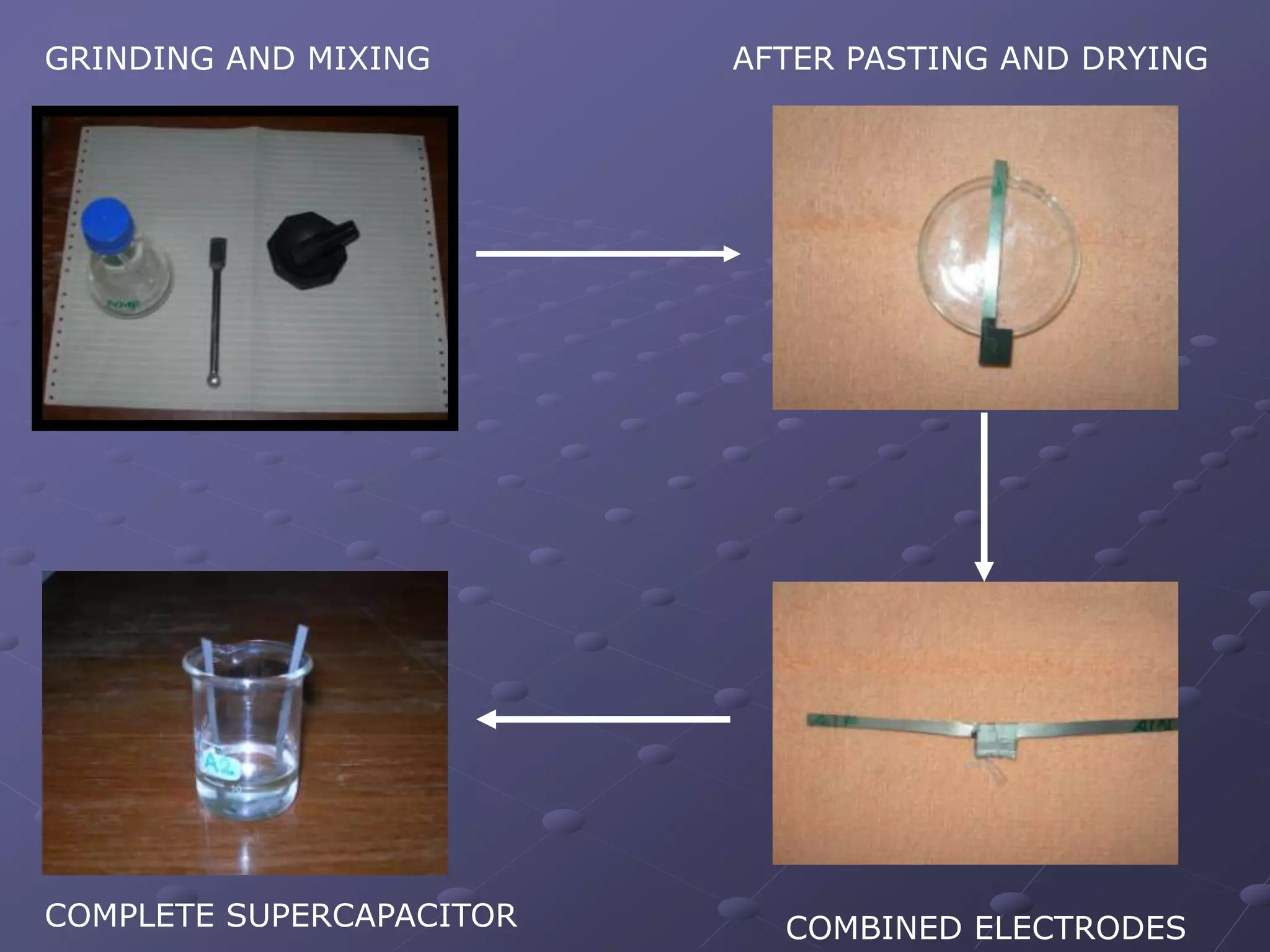
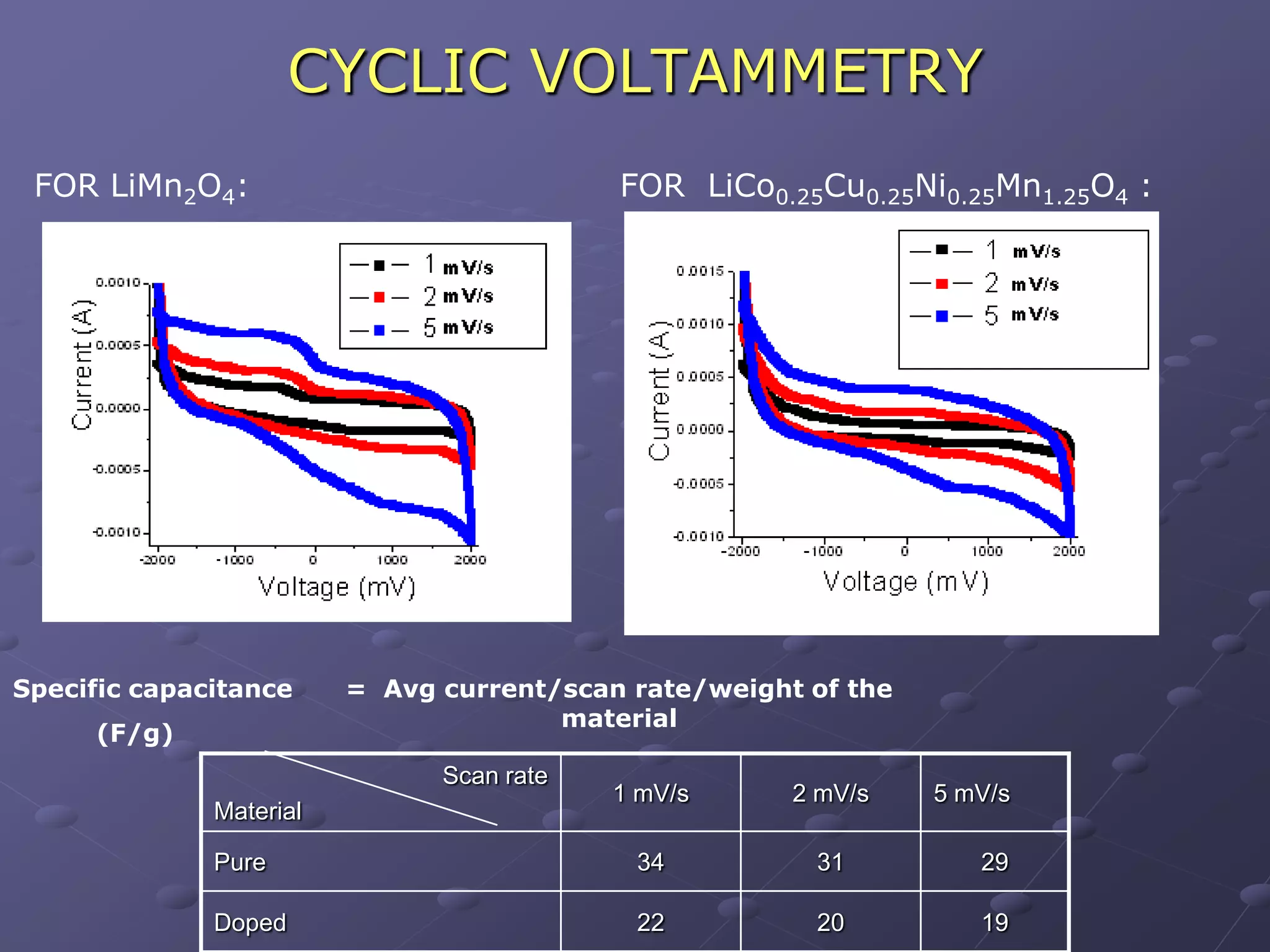
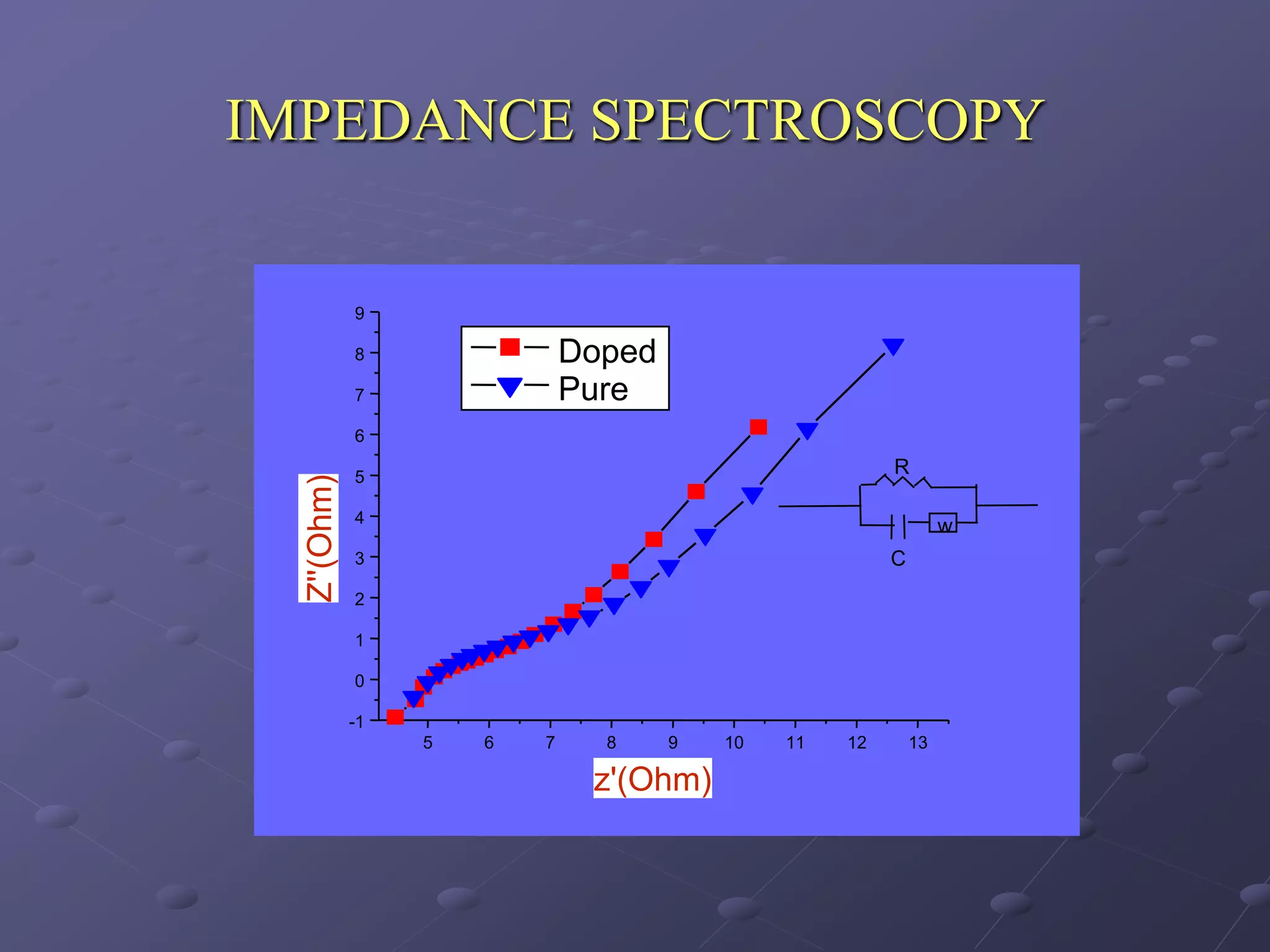
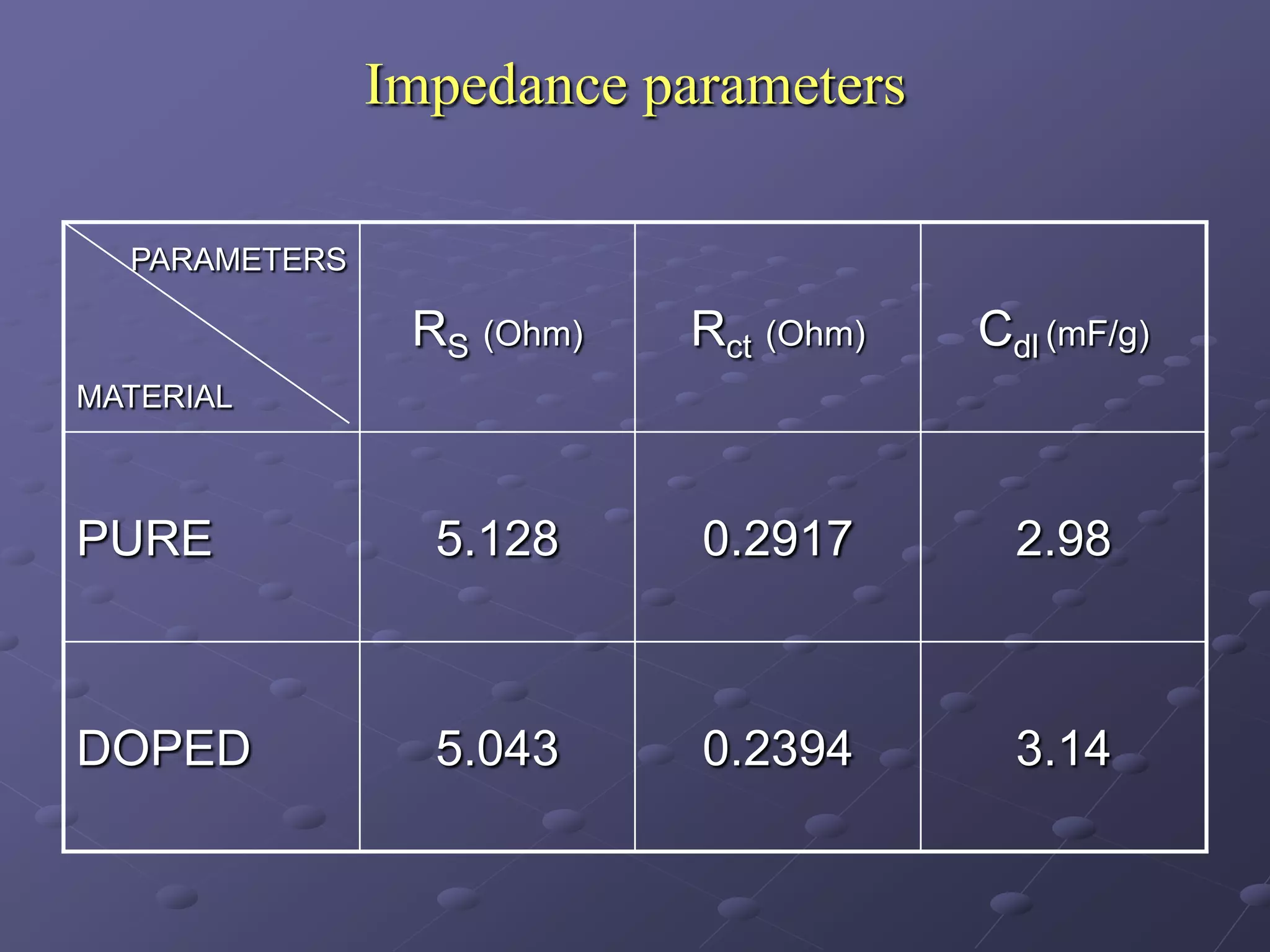
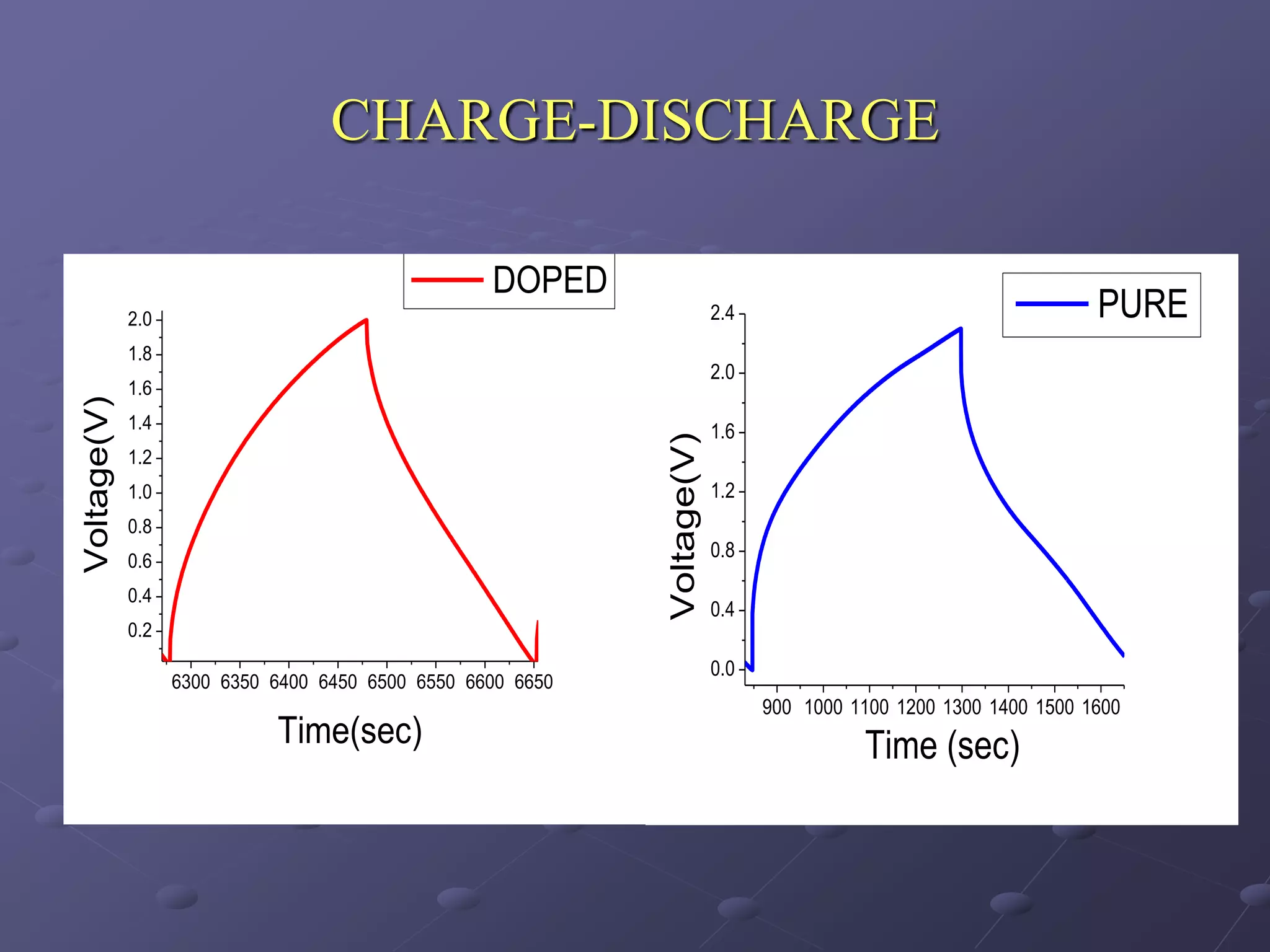
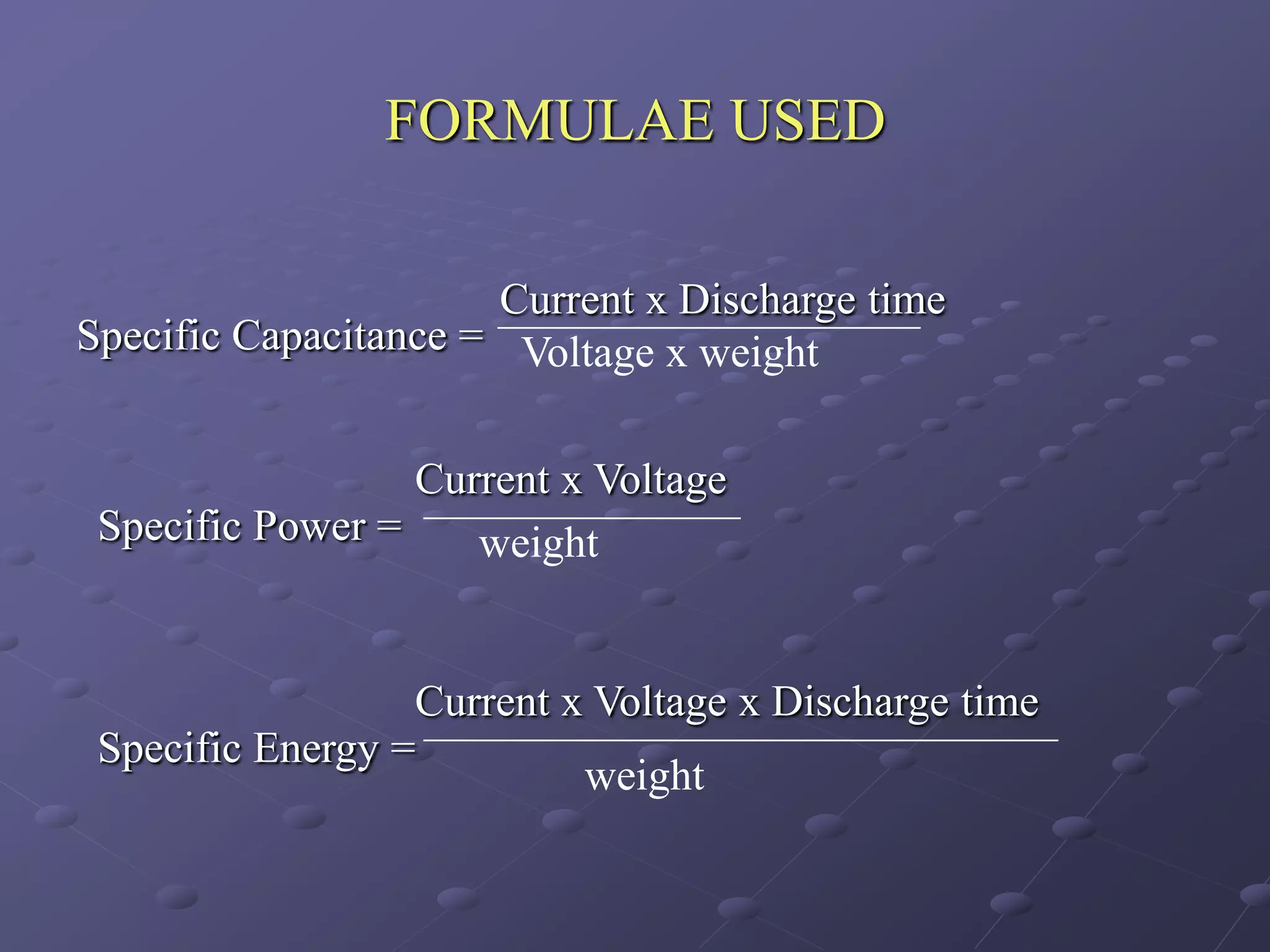
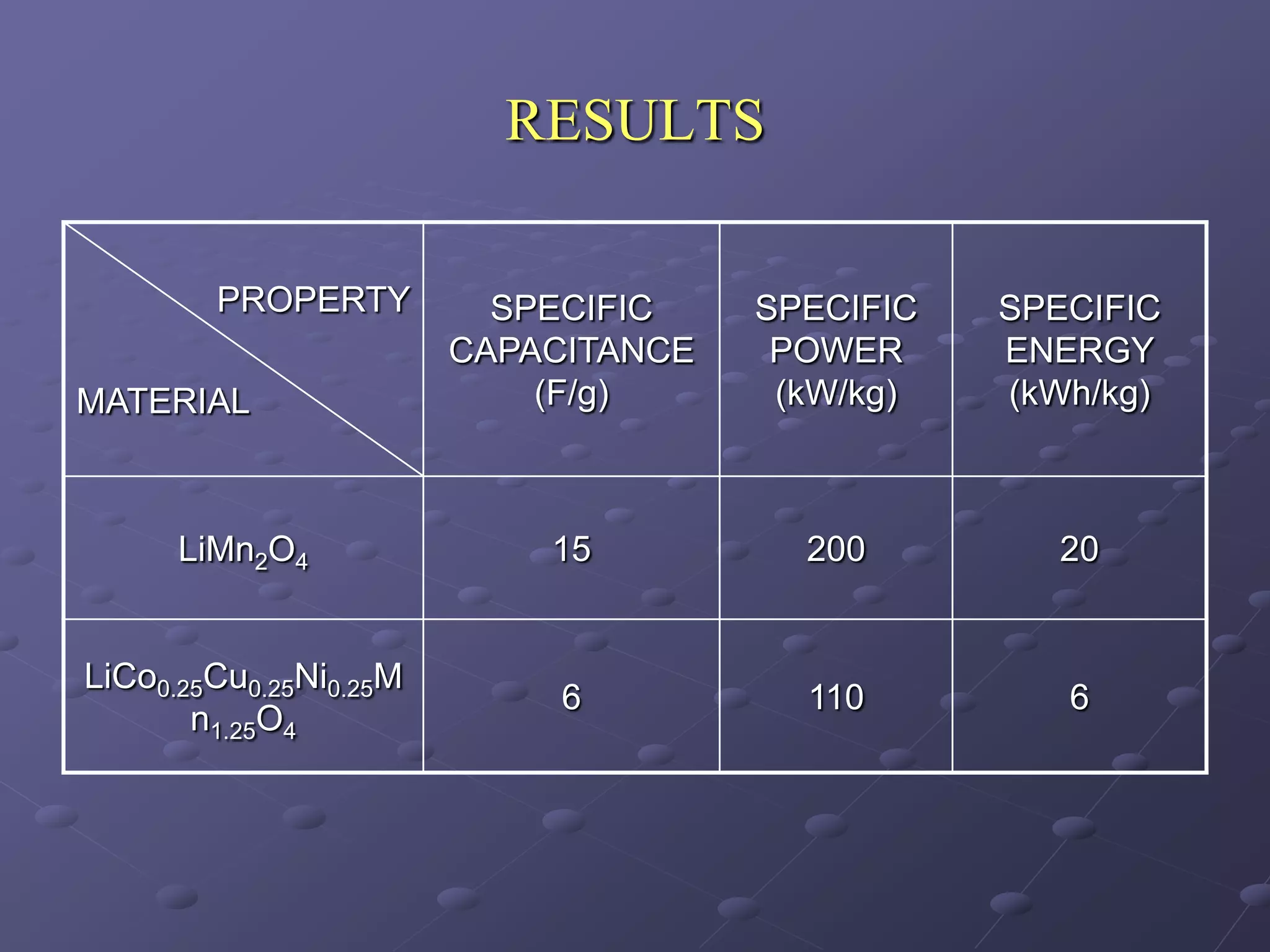
This document describes the development of a hybrid supercapacitor combining lithium-ion battery electrodes and carbon-based supercapacitor electrodes. Specifically, it discusses synthesizing lithium manganate and doped lithium manganate compounds as cathode materials, and using carbon nanofoam as the anode material. The hybrid supercapacitor is fabricated and undergoes electrochemical characterization through impedance spectroscopy, cyclic voltammetry, and galvanostatic charge/discharge testing to analyze its performance.