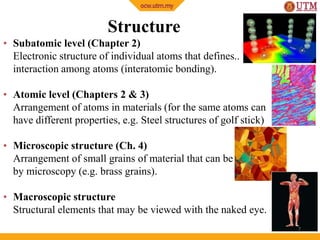
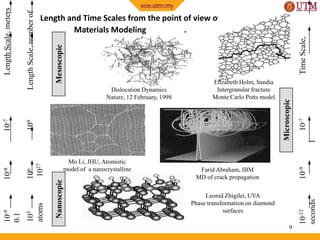
This document provides an introduction to materials science, including definitions, classifications of materials, and historical developments in materials. It discusses the key concepts of processing, structure, properties, and performance as they relate to material science. The four main classes of materials are introduced as metals, ceramics, polymers, and semiconductors. Examples are given to illustrate the structure and wide-ranging applications of these materials classes. The future of materials science is outlined with a focus on nanostructured materials, smart materials, environmentally-friendly materials, and biomimetics.