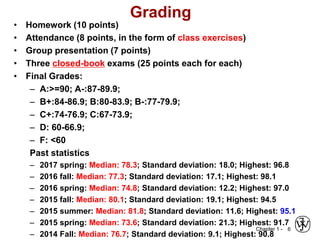
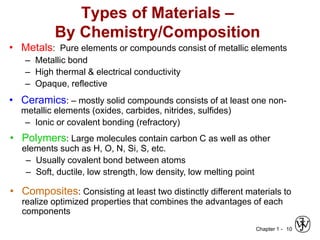
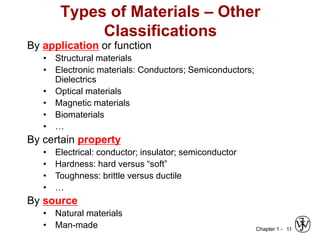
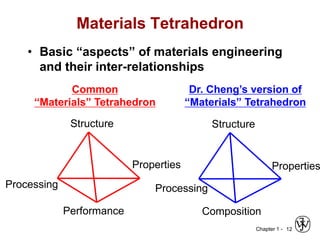
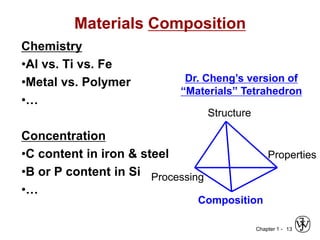
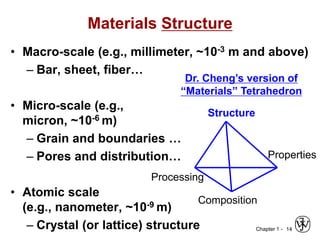
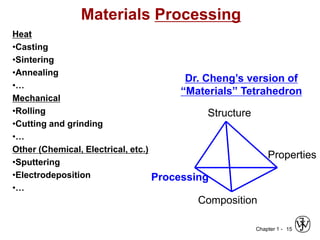
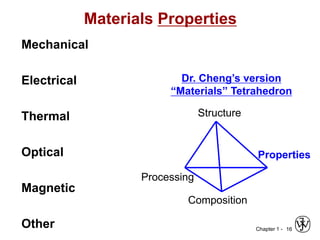
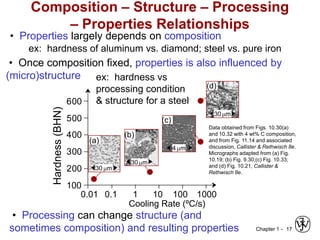
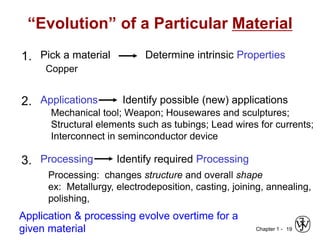
The document outlines the course EGN 3365 in materials engineering, taught by Dr. Zhe Cheng, detailing objectives, prerequisites, grading, policies, and course content such as material properties and classifications. It emphasizes the interdisciplinary nature of the subject and the importance of understanding material selection and processing in engineering applications. Additional resources, including textbooks and grading standards, are also provided.