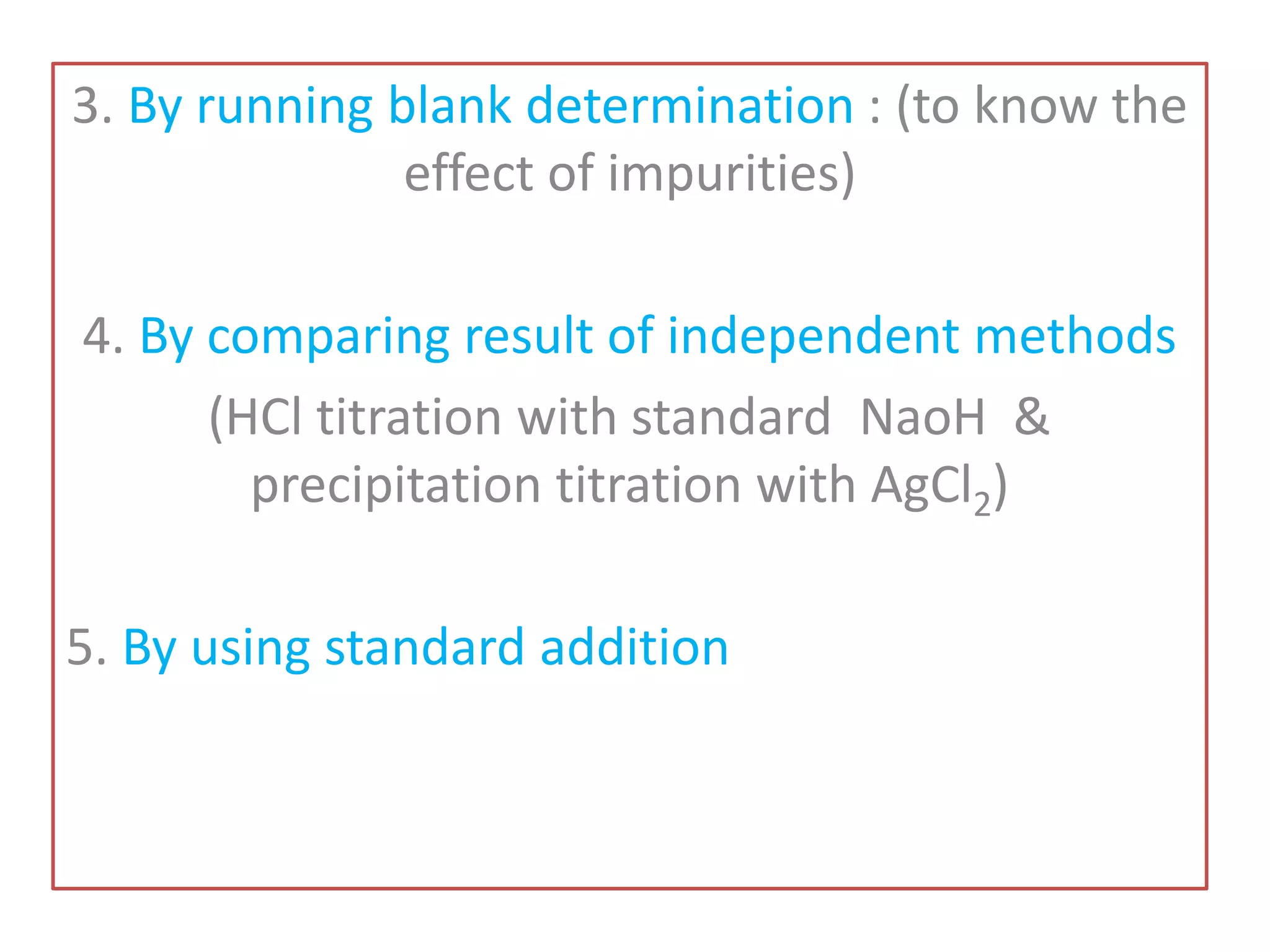
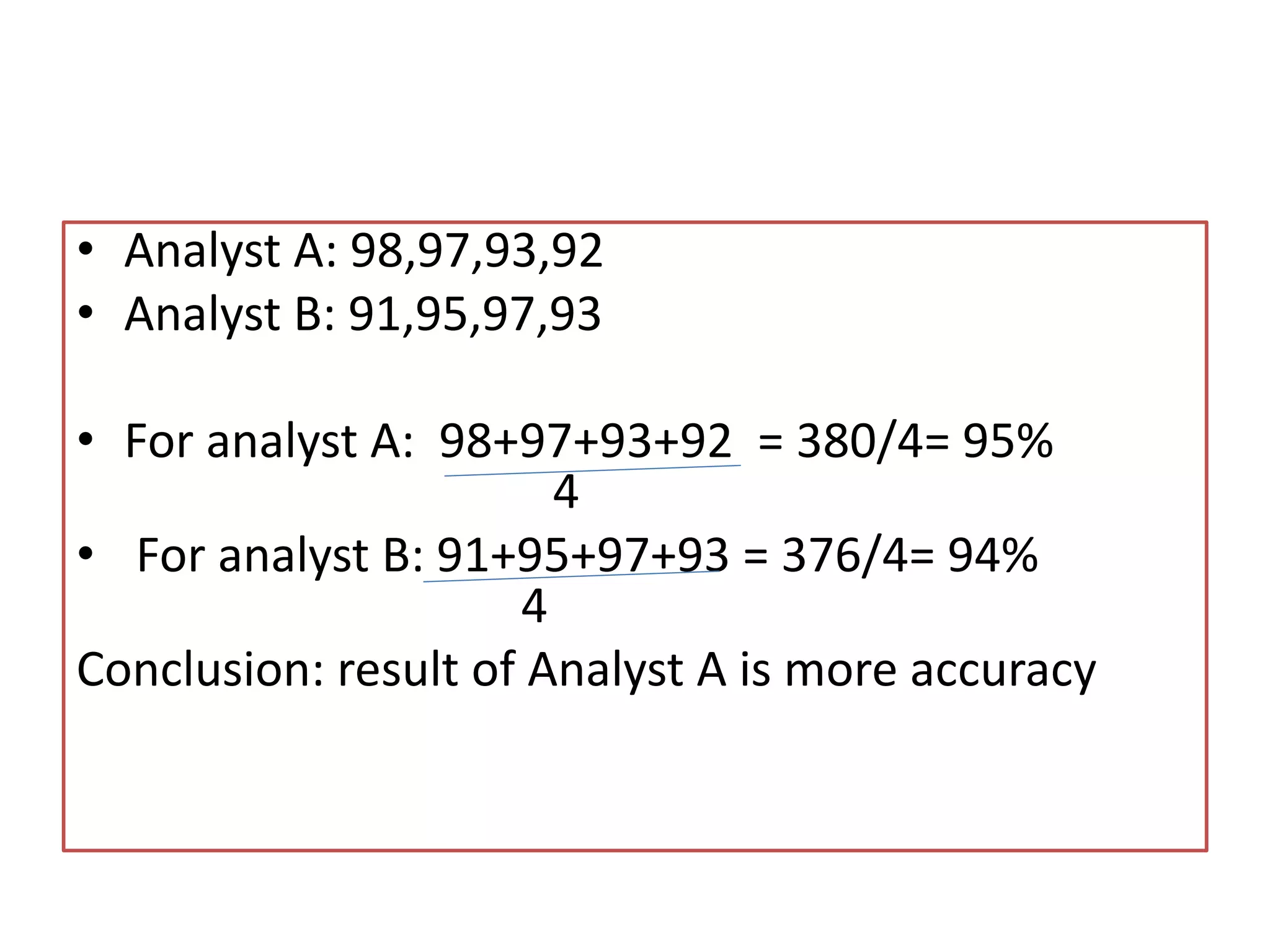
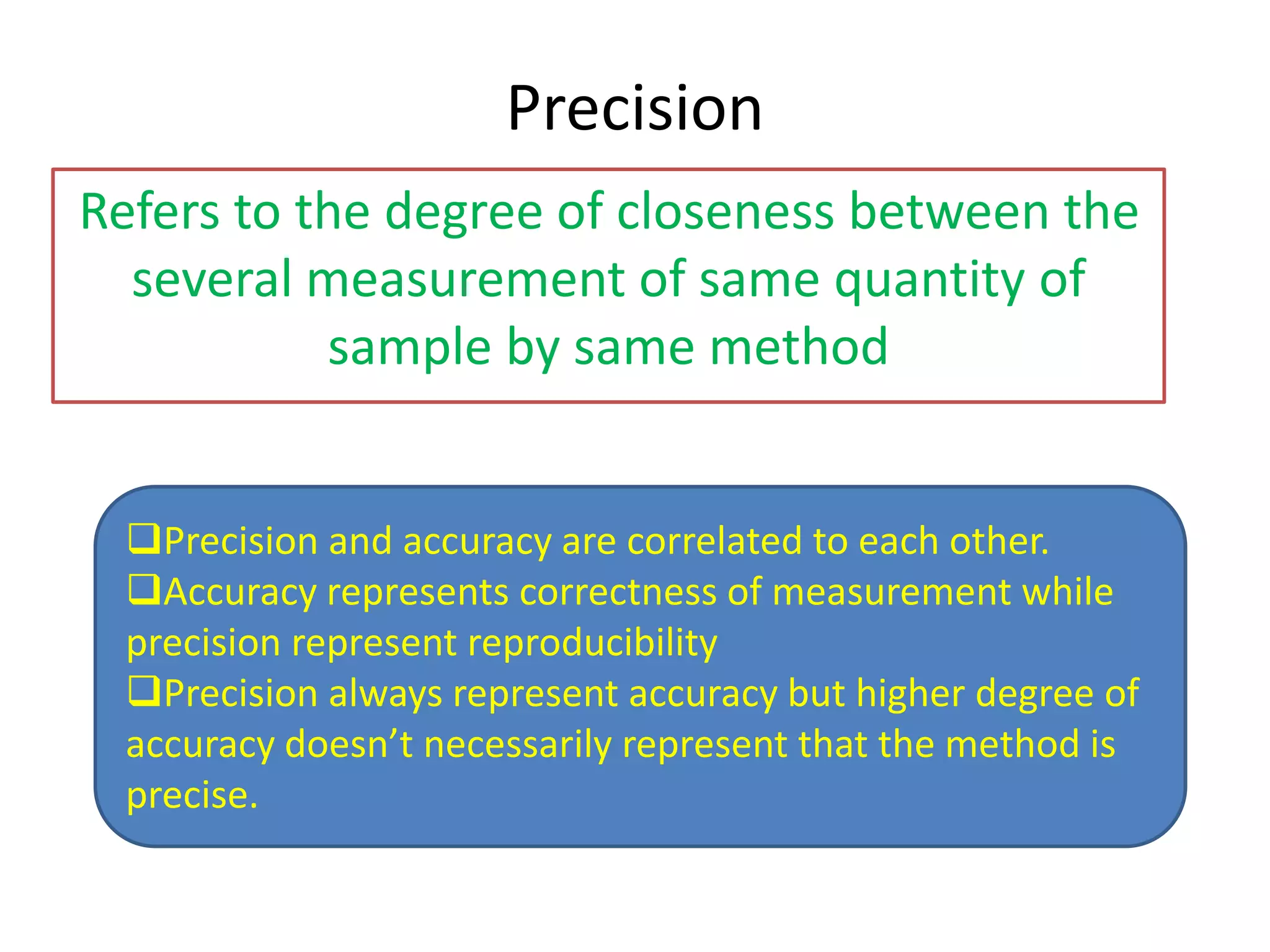
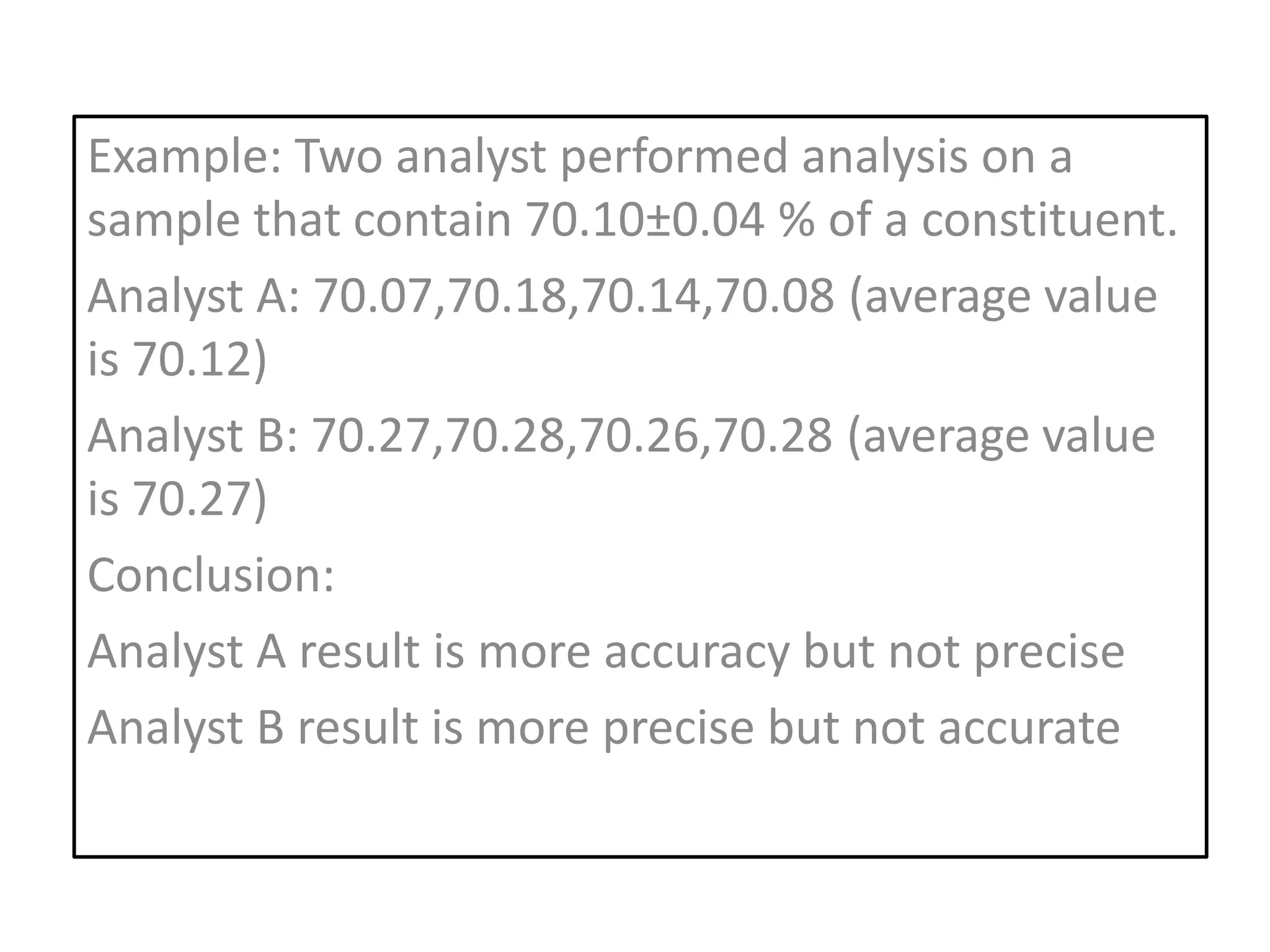
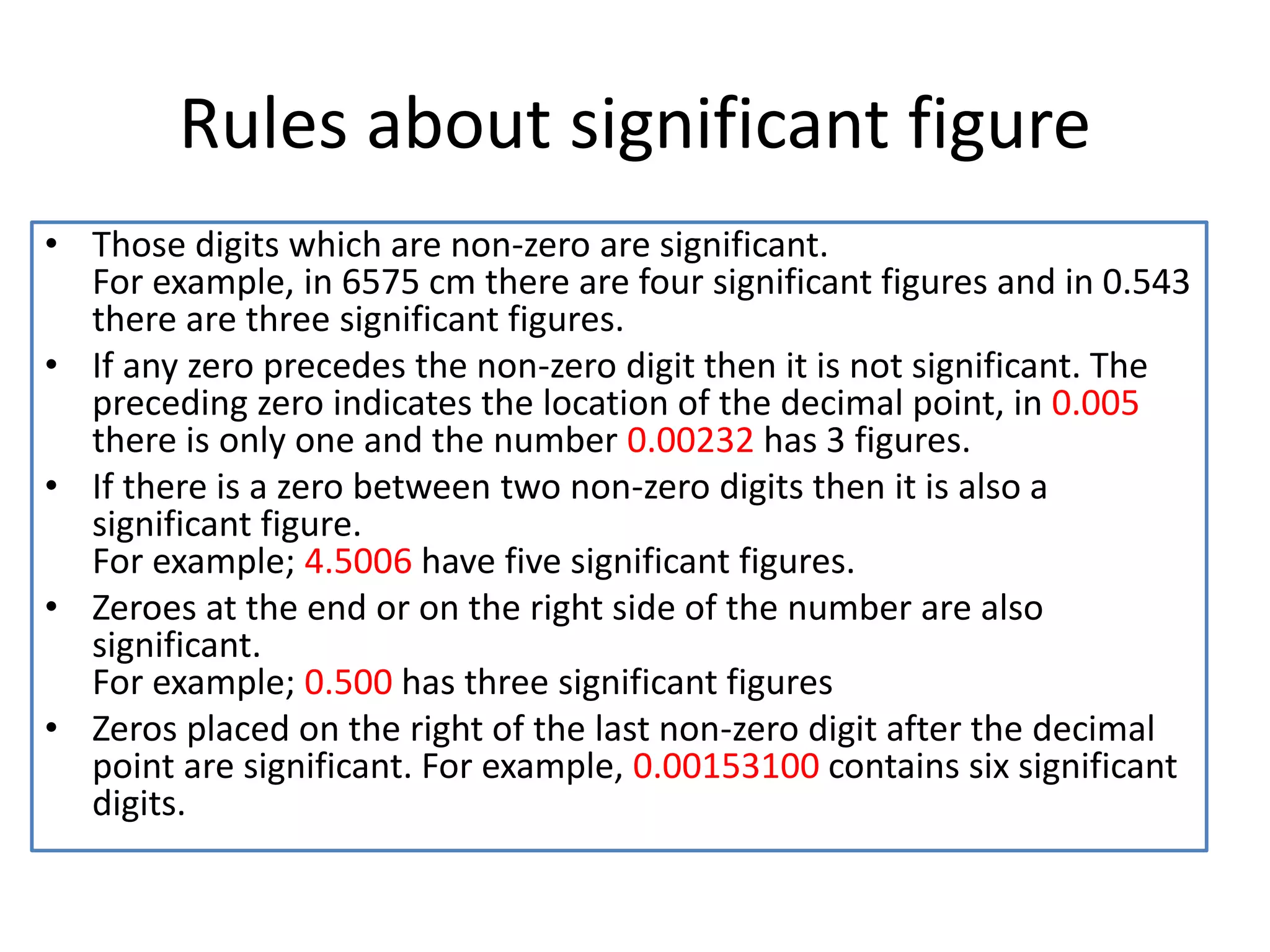
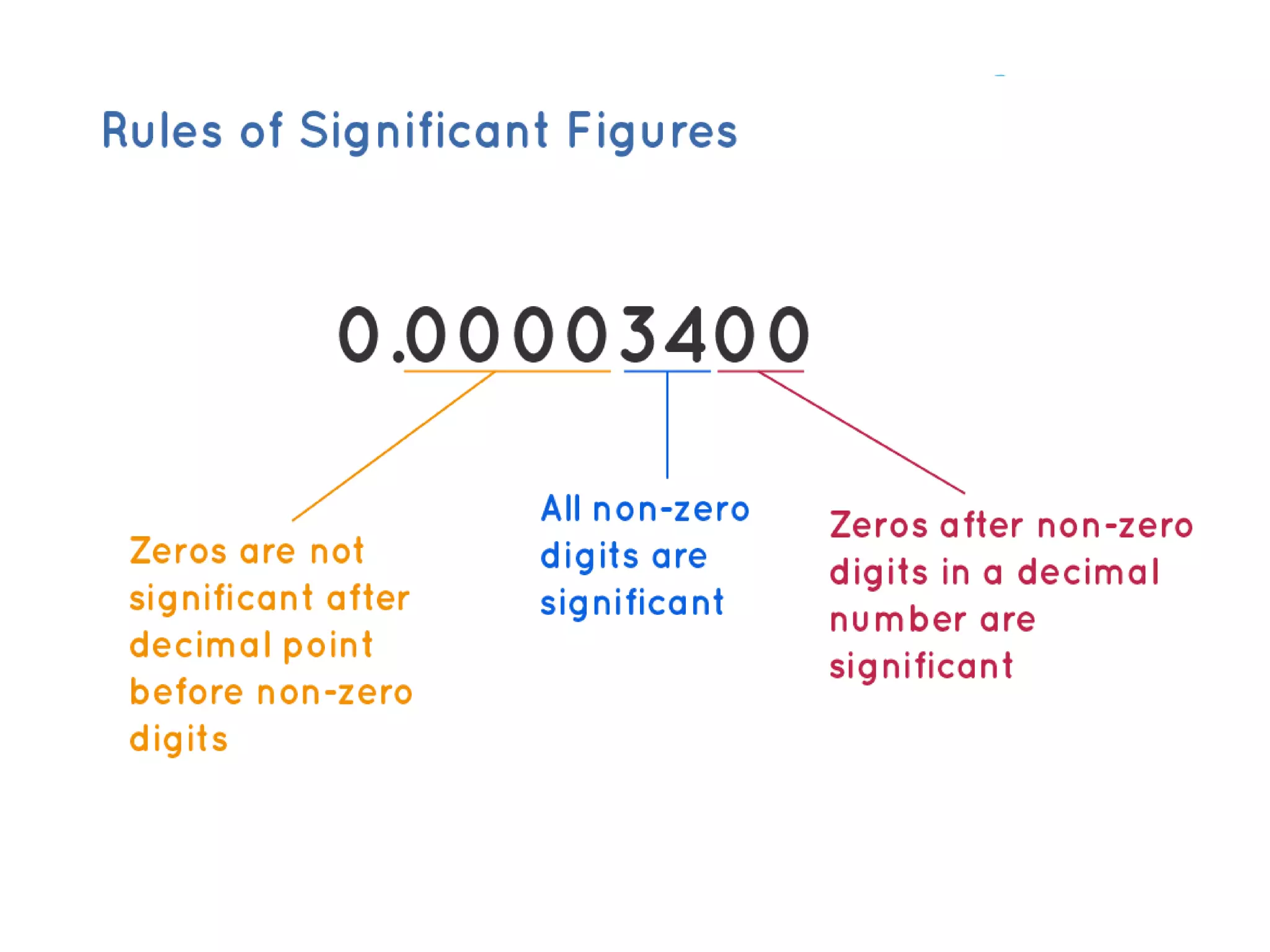
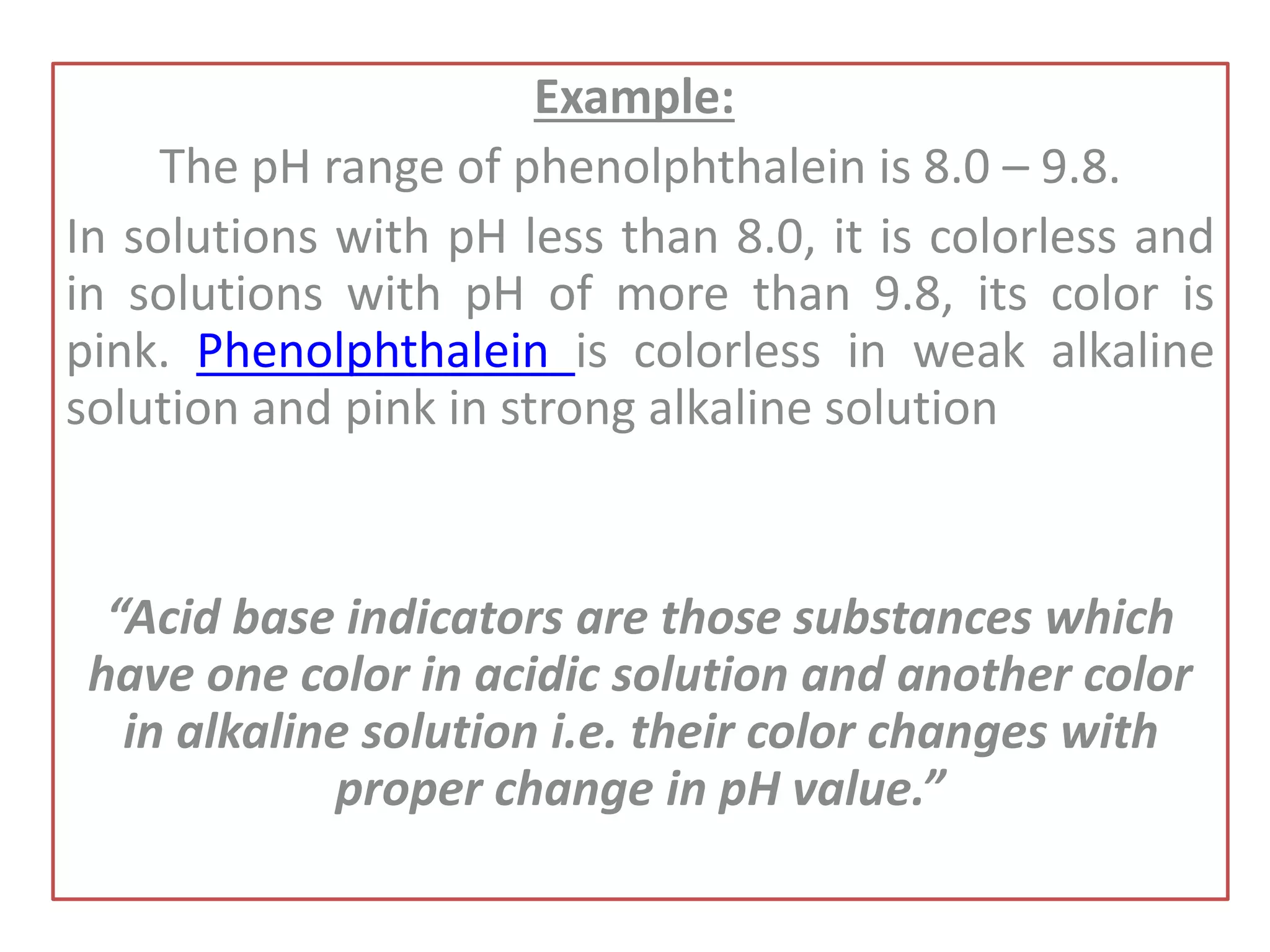
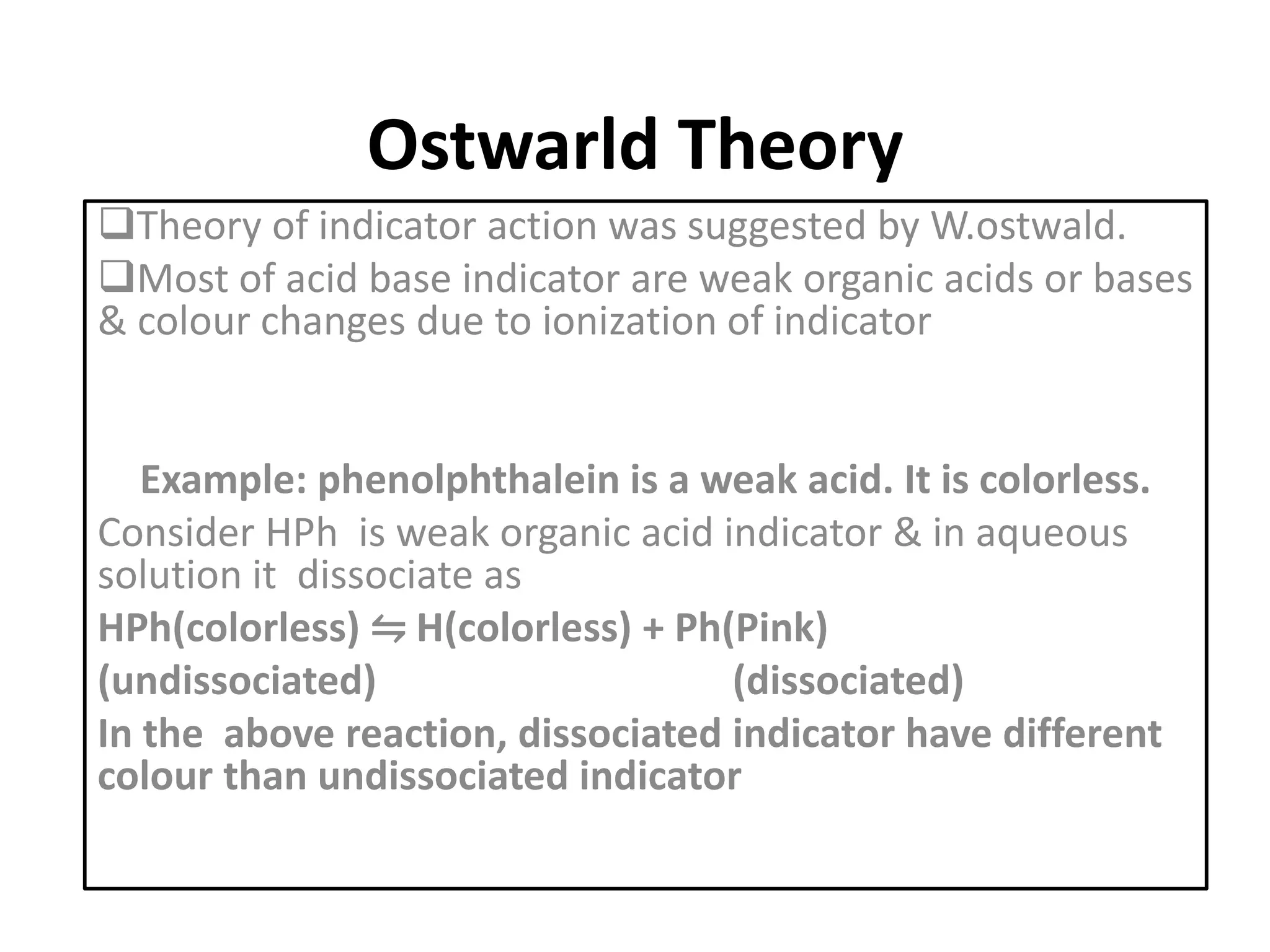
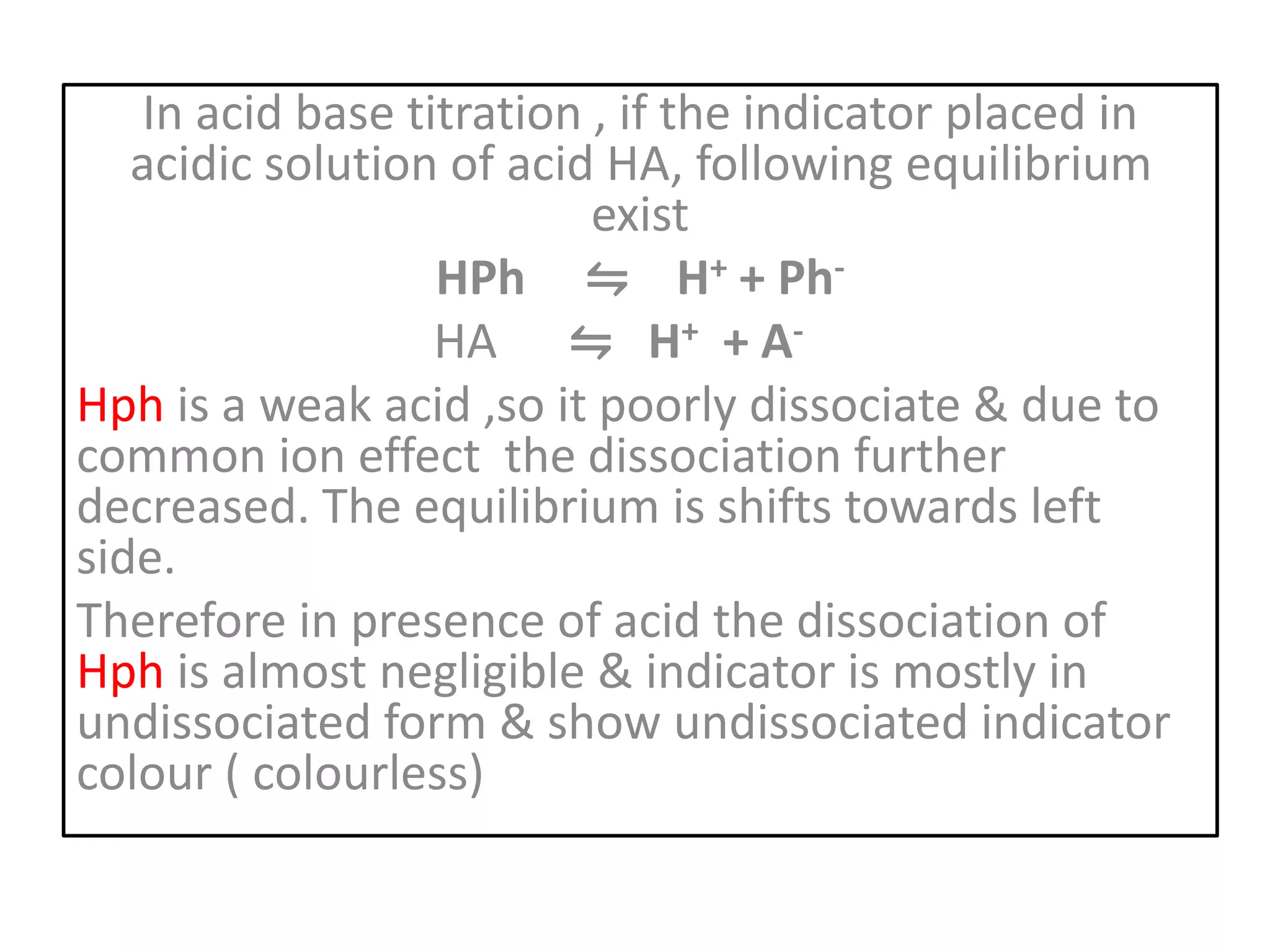
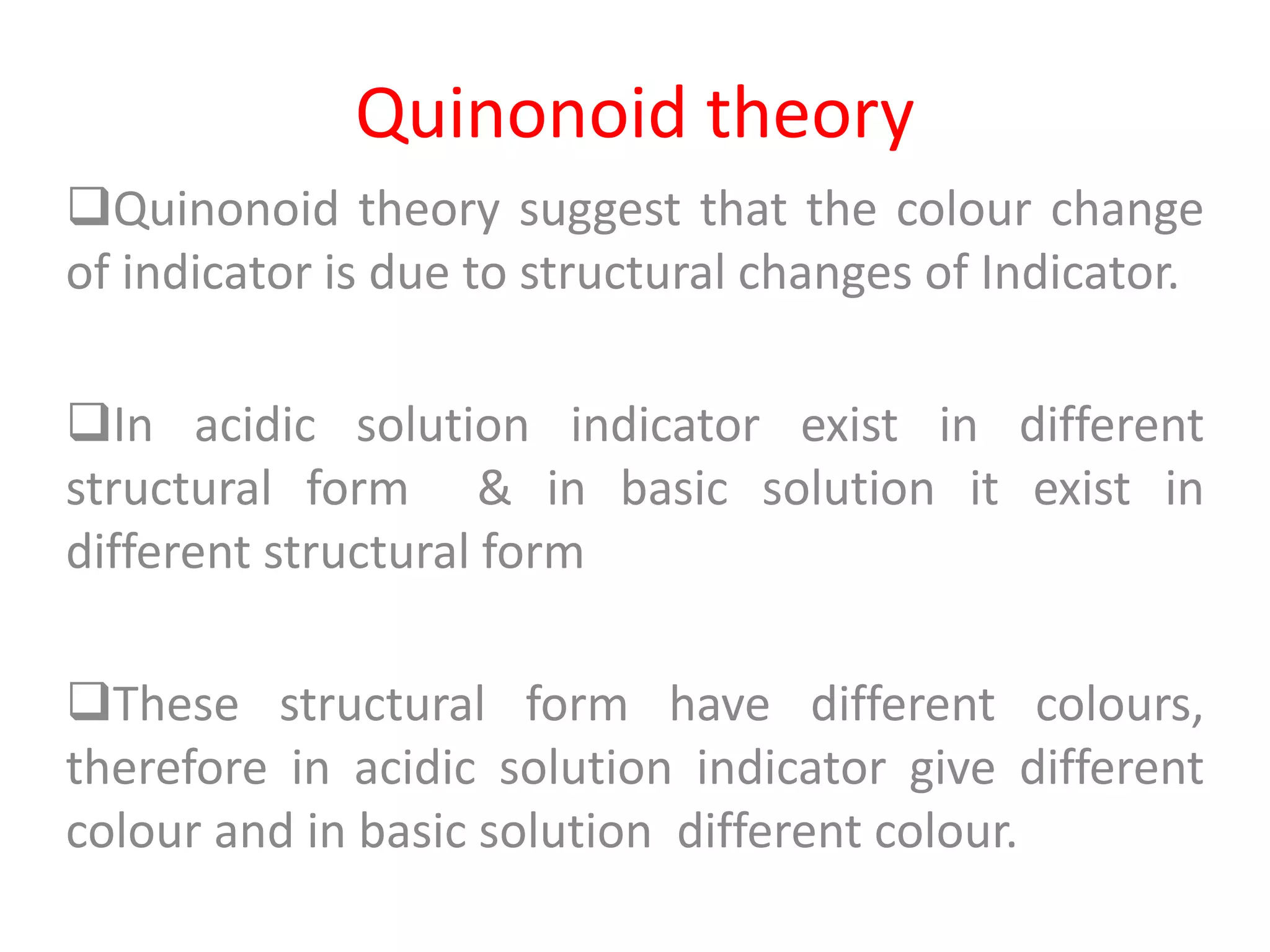
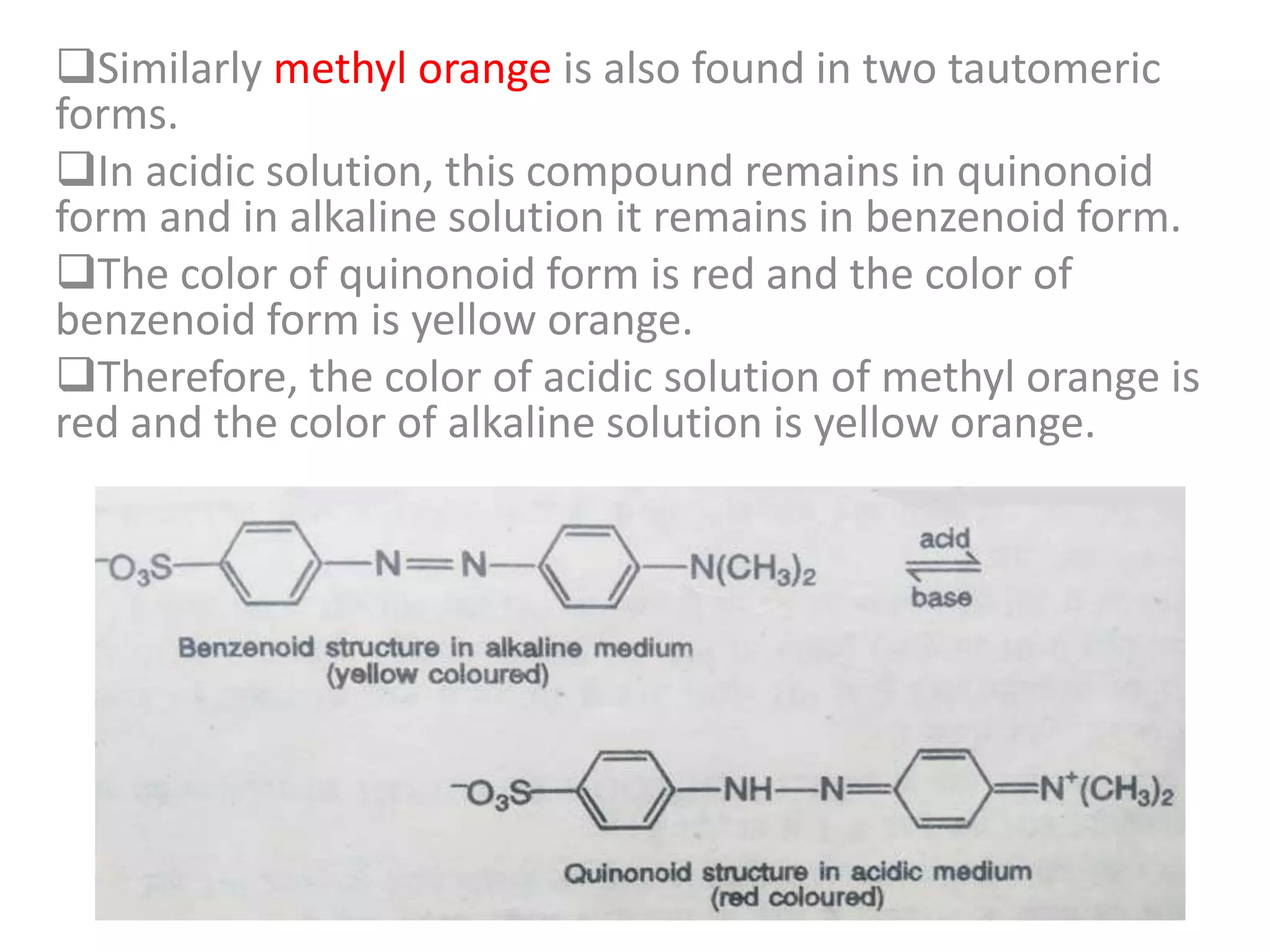
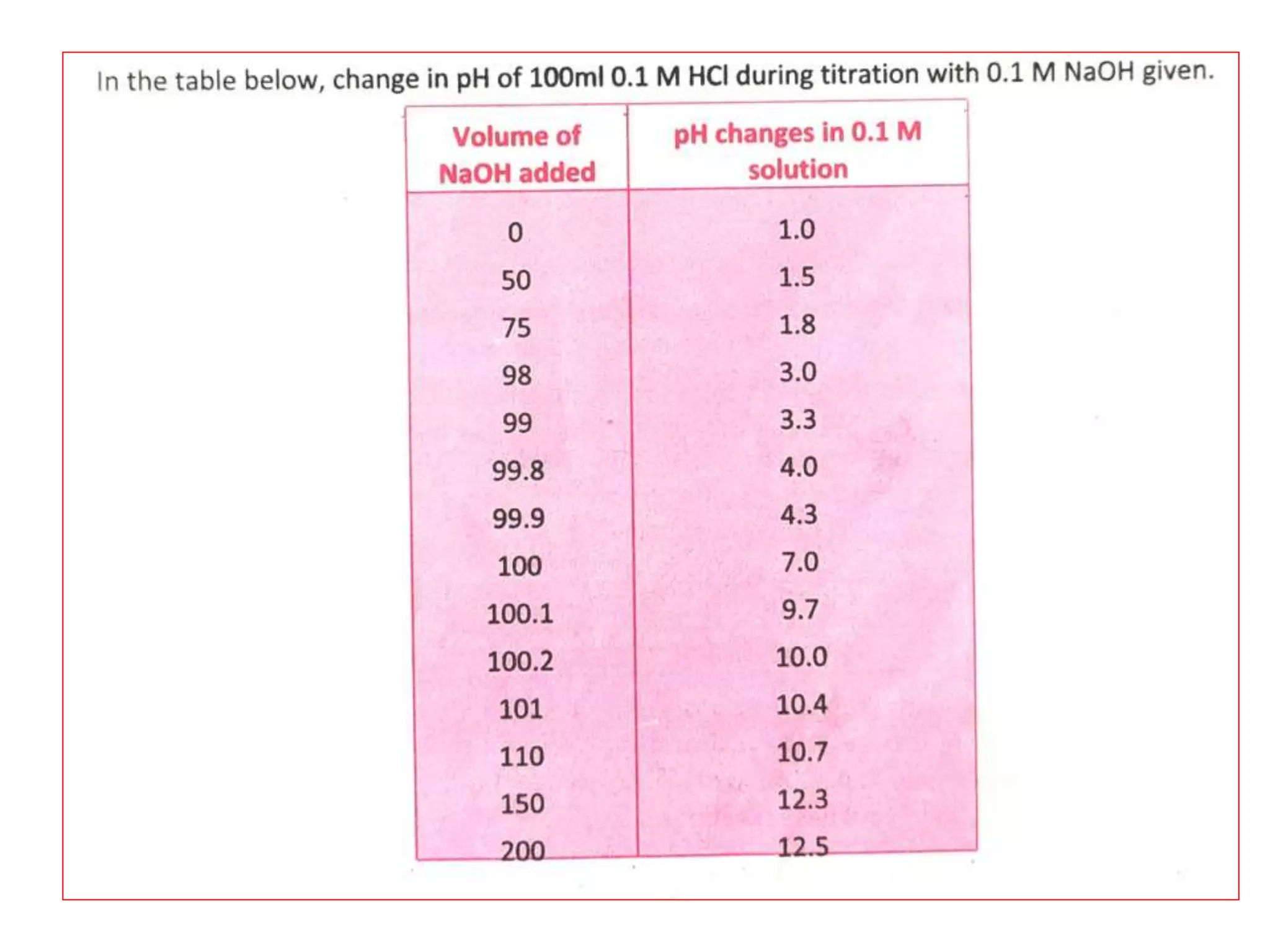
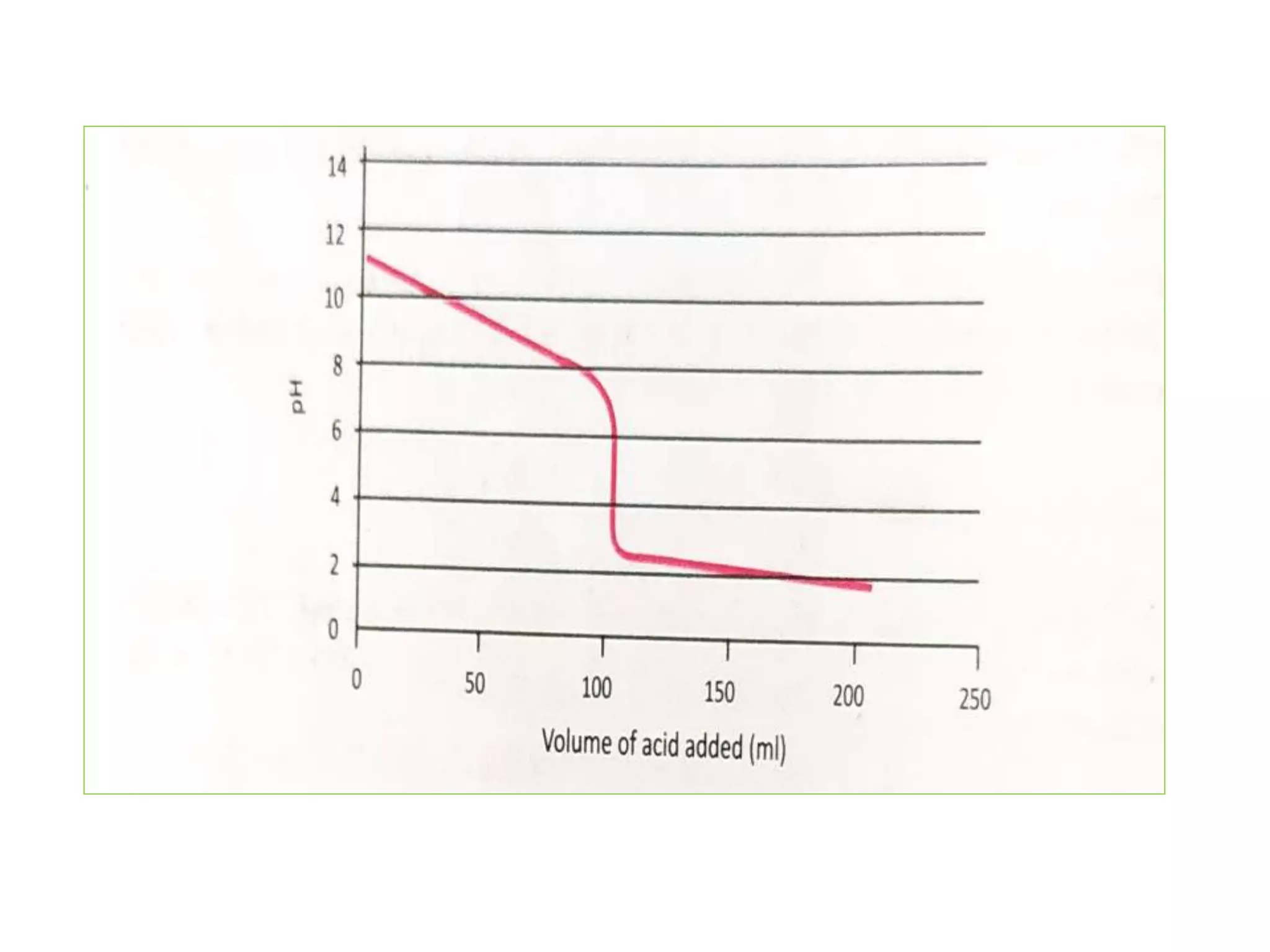
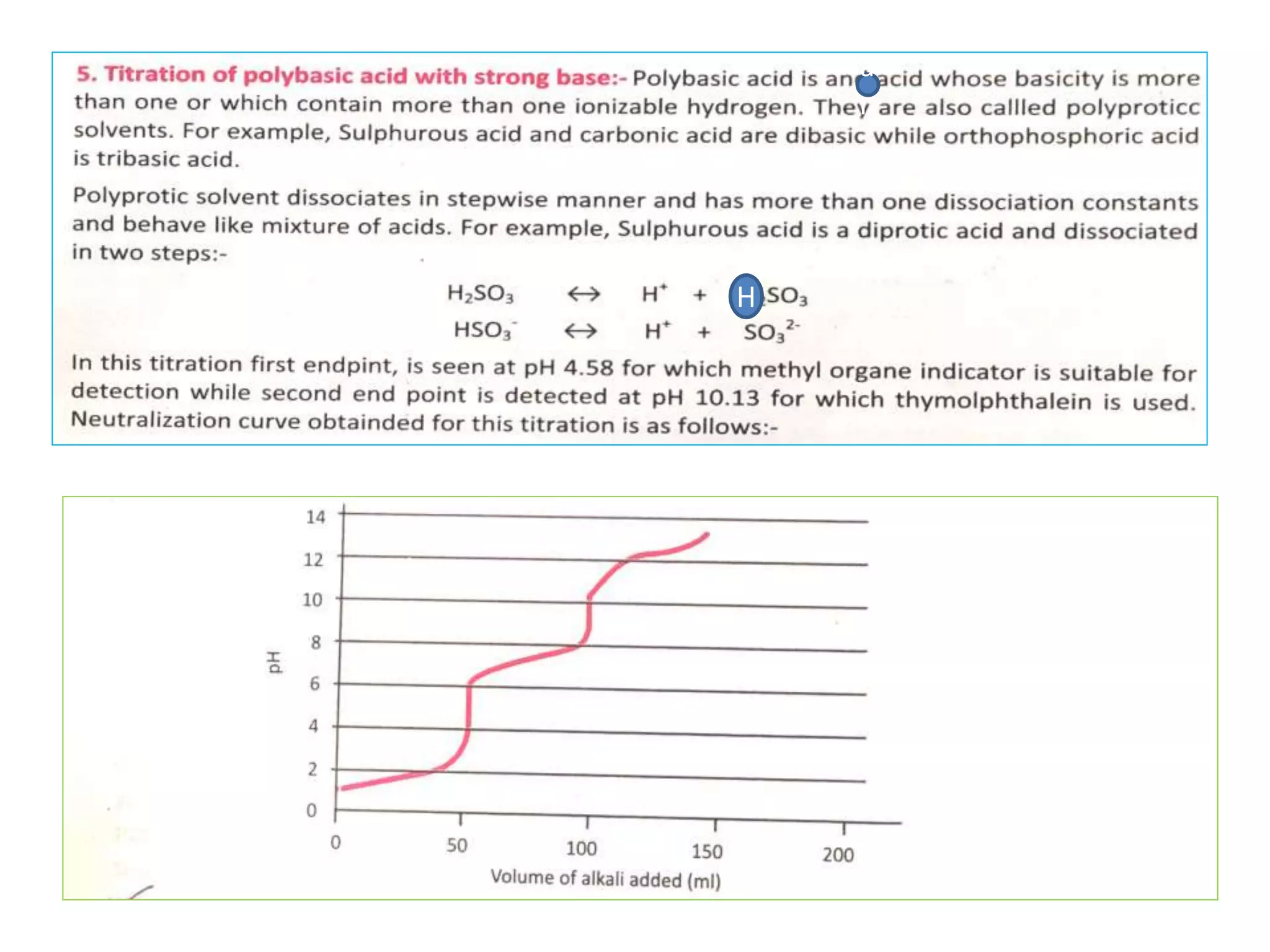
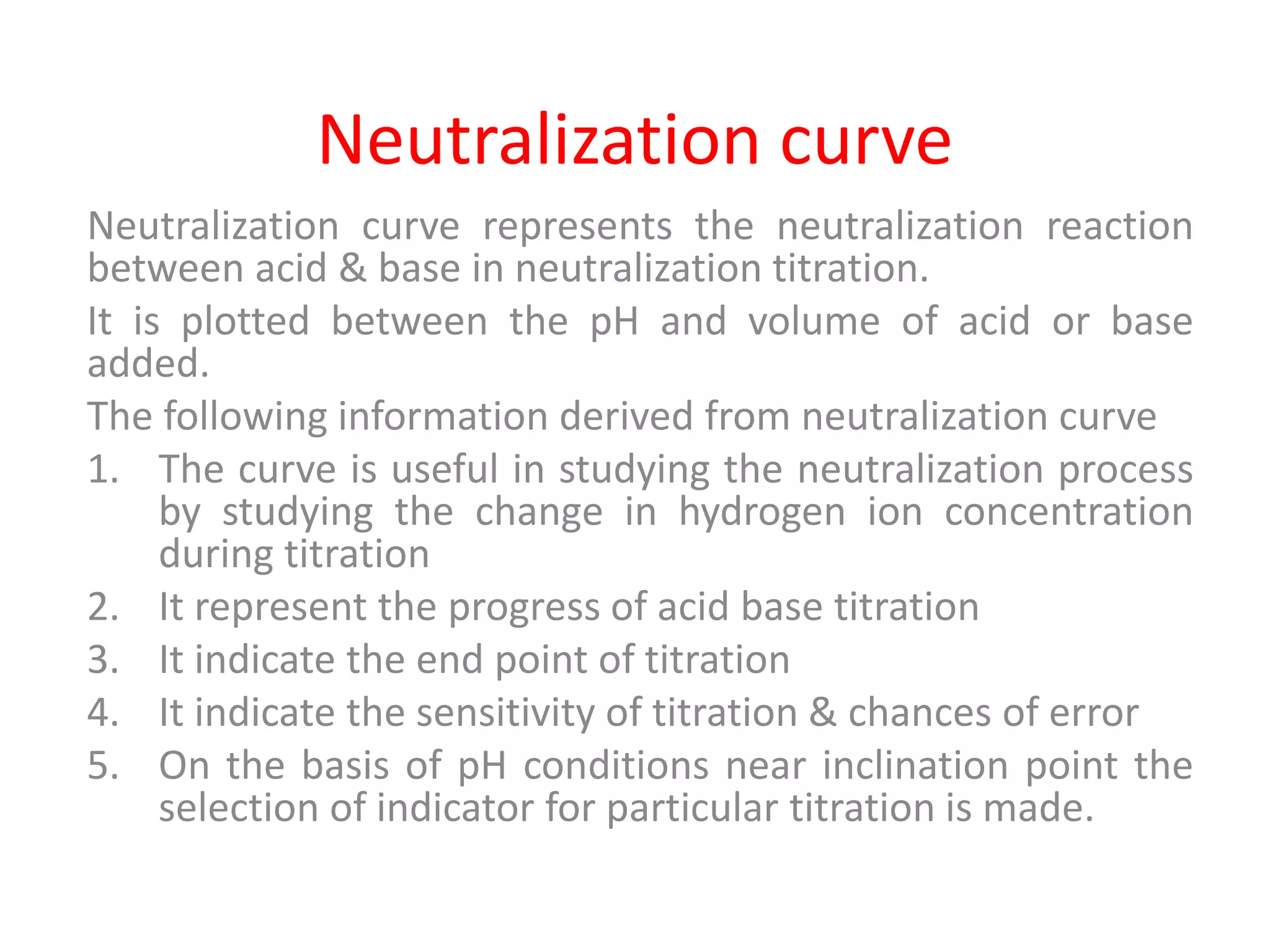
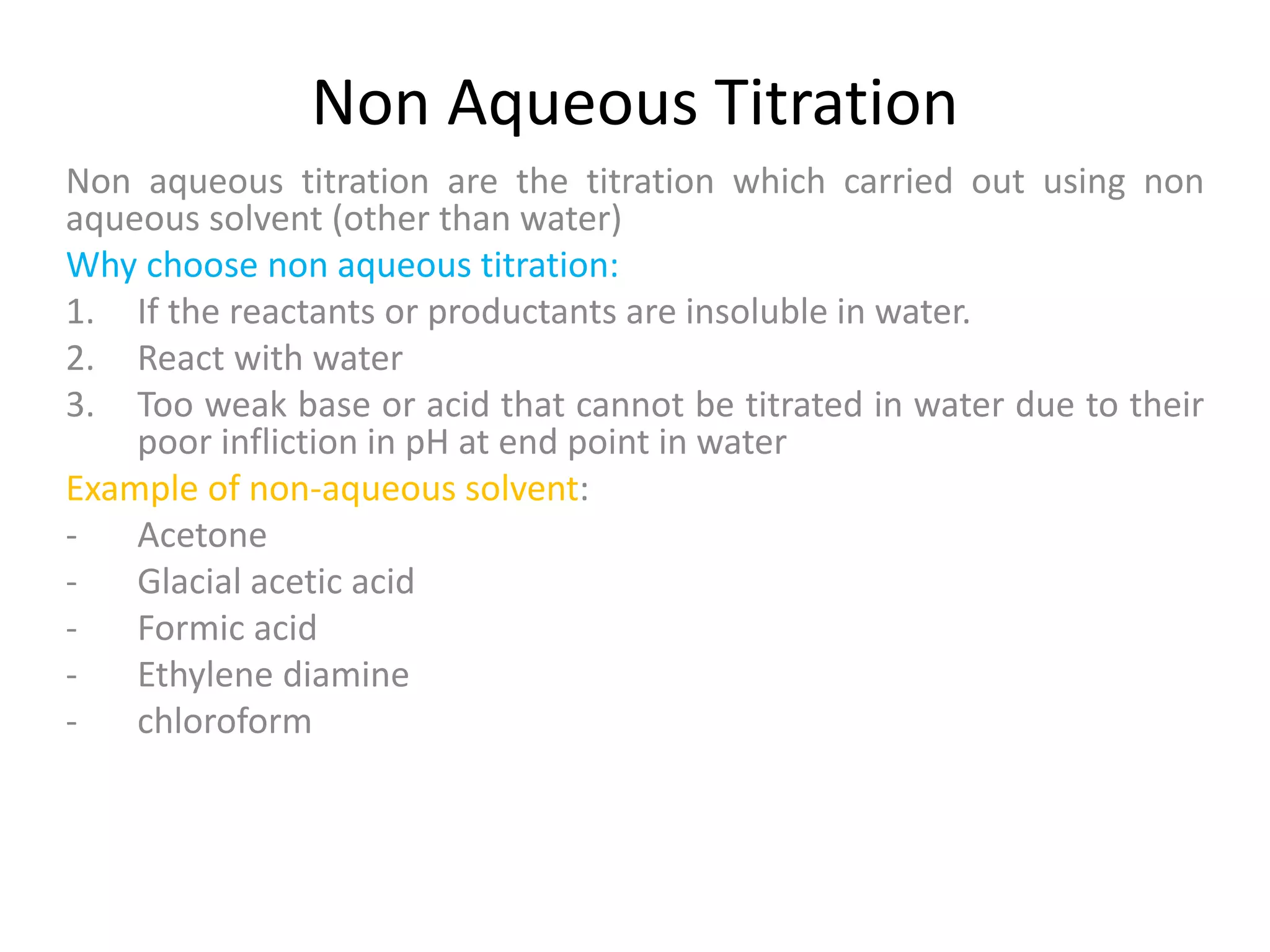
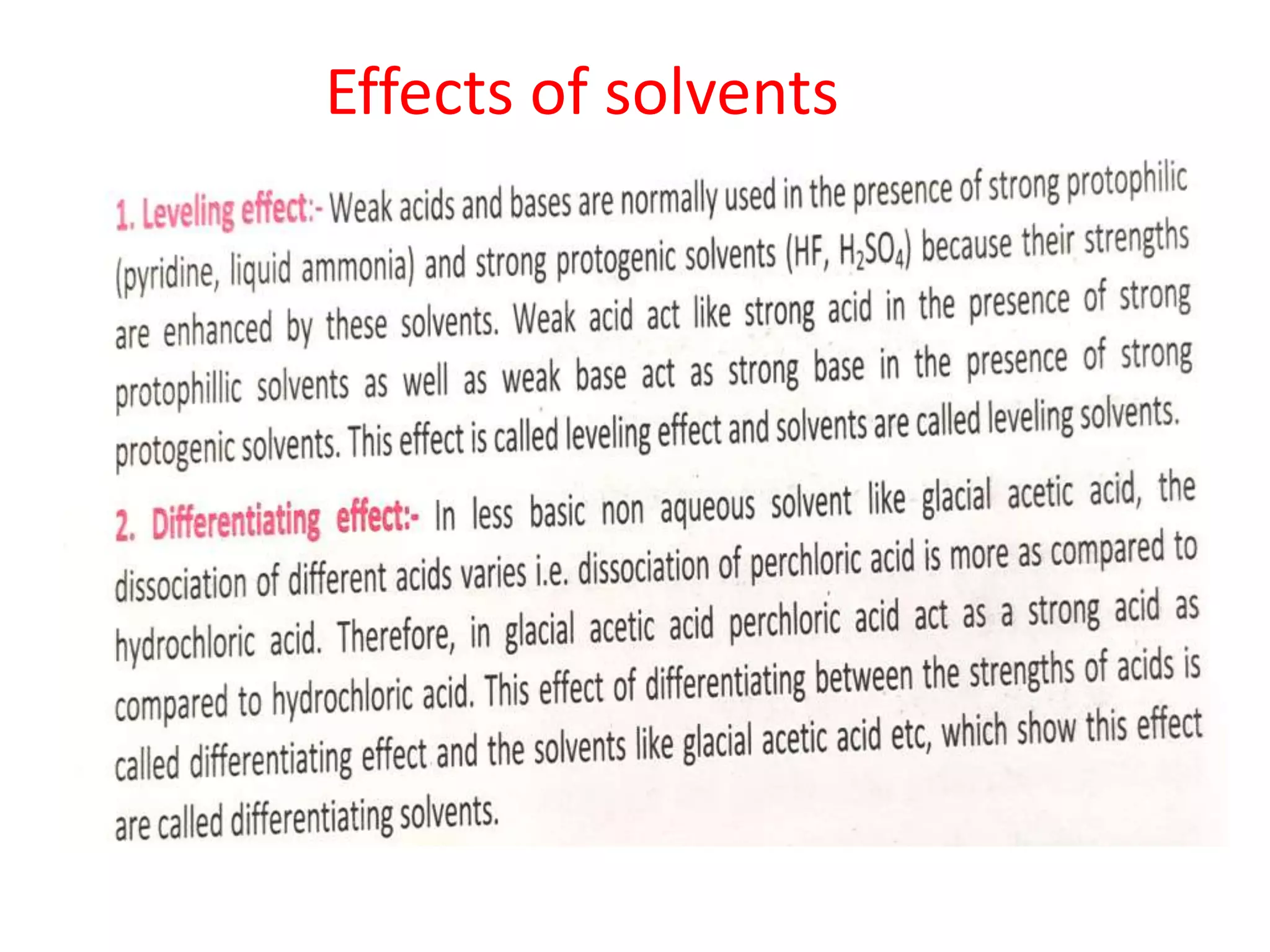
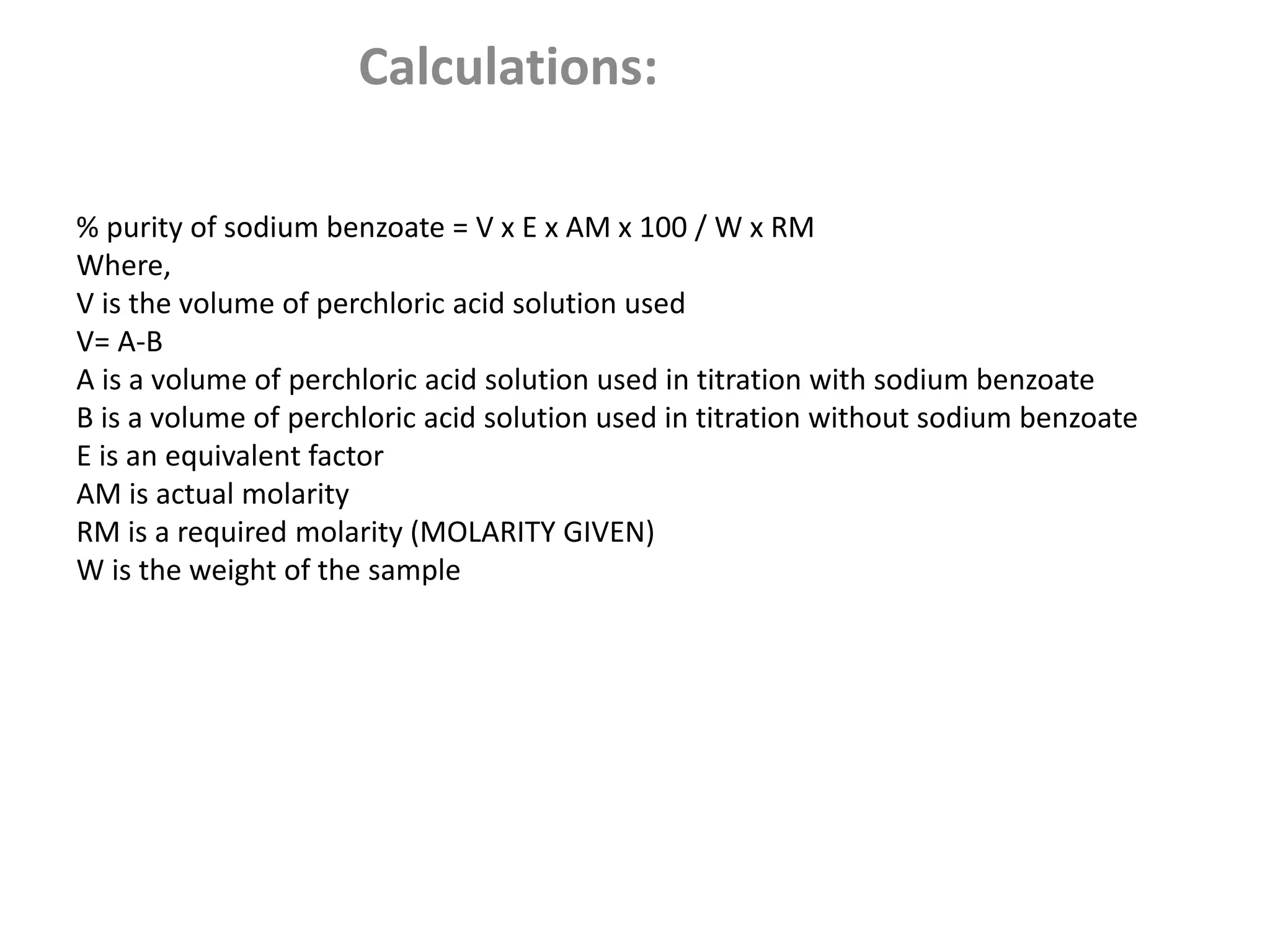
Standardization is the process of determining the concentration of a solution by reacting it quantitatively with a standard solution. Primary standards are highly pure, stable reagents used to standardize and prepare secondary standards. They have properties like high purity, stability, and molecular weight. Primary standards like sodium chloride and sodium carbonate are used to standardize silver nitrate and acid solutions respectively. Accurate standardization is important for titration calculations which rely on knowing the true concentration of the standard solution.